National Center for Global Health and Medicine
Xenotransplantation of a novel immune-isolation device encapsulating porcine islets improved blood glucose levels in diabetic common marmosets without immunosuppressive drugs
Kumiko Ajima1, Naoto / Tsuda2, Shigeki / Matsumoto2, Masayuki / Shimoda1.
1Pancreatic Islet Cell Transplantation Project, Research Institute National Center for Global Health and Medicine, Tokyo, Japan; 2Biomaterials Business Division, Mochida Pharmaceutical Co., Ltd., Gotemba, Japan
Introduction: Islet transplantation is an effective treatment for type 1 diabetes (T1D), but donor shortages and the need for immunosuppressants are major challenges. Porcine islets are a promising cell resource for diabetes treatment. However, delivery of these islets, control of the xenogeneic immune response, maintenance, and retrieval of transplanted islets remain a challenge. We have previously developed a novel device with immune-isolation ability for xenotransplantation, and have published that the device encapsulating porcine islets can improve glycemic control for more than 200 days without immunosuppressive drugs when transplanted in immunocompetent diabetic mice (2022 Cell Rep. Methods. K. Ajima et al.) We then systematically evaluated this xenotransplantation device in larger animal models than mice: nonhuman primates.
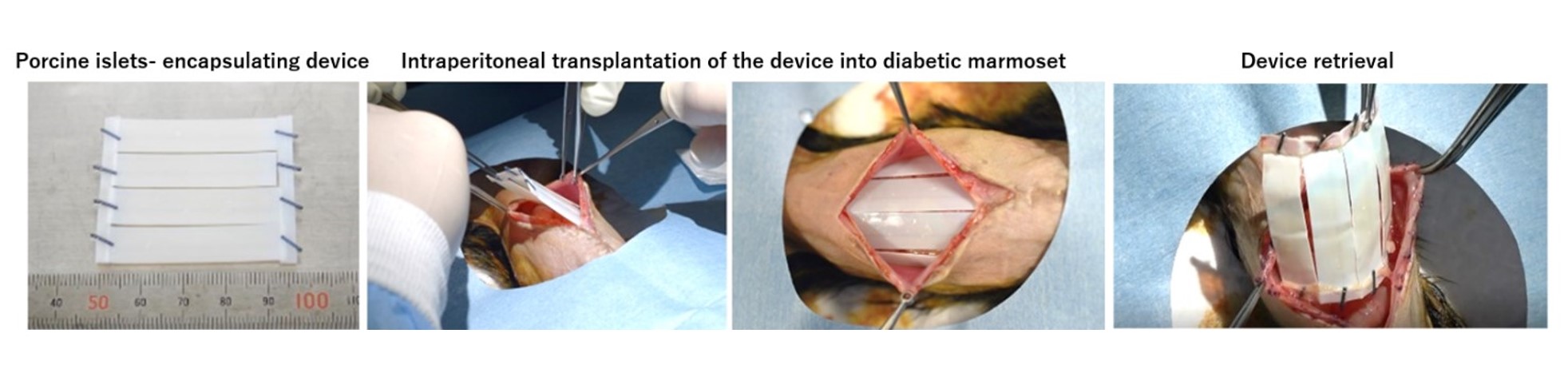
Method: In this study, we created a diabetic model of common marmoset by pancreatic resection and intravenous administration of streptozotocin and then intraperitoneally transplanted the devices encapsulating porcine islets. Anti-inflammatory drugs, etanercept, tofacitinib were administered to some of them, but no immunosuppressive drugs were used (n=7). The transplantation device consists of a chemically crosslinked alginate gel and a semipermeable cellulose membrane (MWCO 100 kDa) with immunoisolation ability, and is both flexible and durable. Porcine islets were arranged regularly near the surface inside the sheet hydrogel. Porcine islets were transplanted 40,000 IEQ per animal (A diabetic marmoset weighs about 300g, n=7). The shape of the device is a sheet about 4.5 cm square, in which four devices of 1 x 4cm (including 10,000 IEQ) are connected in parallel. The device was transplanted without fixation between the peritoneal viscera and the abdominal wall (Figure 1). Each marmoset was observed for up to 12weeks (w).
Results: Of the individuals who survived more than 3w, most showed improvement in glycemic control, with improvement in either blood glucose, hemoglobin A1c (HbA1c), or oral glucose tolerance test compared to pre-transplantation. The average non-fasting blood glucose levels of the 4 marmosets observed up to 12w were 363±90 mg/dL before transplantation, 265±56 mg/dL (4w after TX), and 284±48 mg/dL (12w after TX). Furthermore, each HbA1c of the 4 marmosets observed up to 12w decreased from 9.3% to 7.2%, 6.8% to 6.1%, 6.1% to 5.0%, and 7.0% to 5.5% (6w after TX). The device was retrieved after the observation period and was easily retrieved without almost no adhesions or major fibrosis (Figure 1). No adverse effects on the devices and marmosets were seen with anti-inflammatory drug administration.
Conclusion: These results demonstrate the potential of the porcine islet-encapsulating device in the treatment of diabetes in preclinical studies.
Figure1
This work was partially supported by research funds from Mochida Pharmaceutical Co., Ltd..
References:
[1] K. Ajima et al., Cell Reports Methods.2022; 3: 100370.
Lectures by Masayuki Shimoda
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Thu-26 14:45 - 15:45 |
Islet Xeno: Preserving graft integrity and evaluating the implant site | Xenotransplantation of a novel immune-isolation device encapsulating porcine islets improved blood glucose levels in diabetic common marmosets without immunosuppressive drugs. | Indigo 204 |
