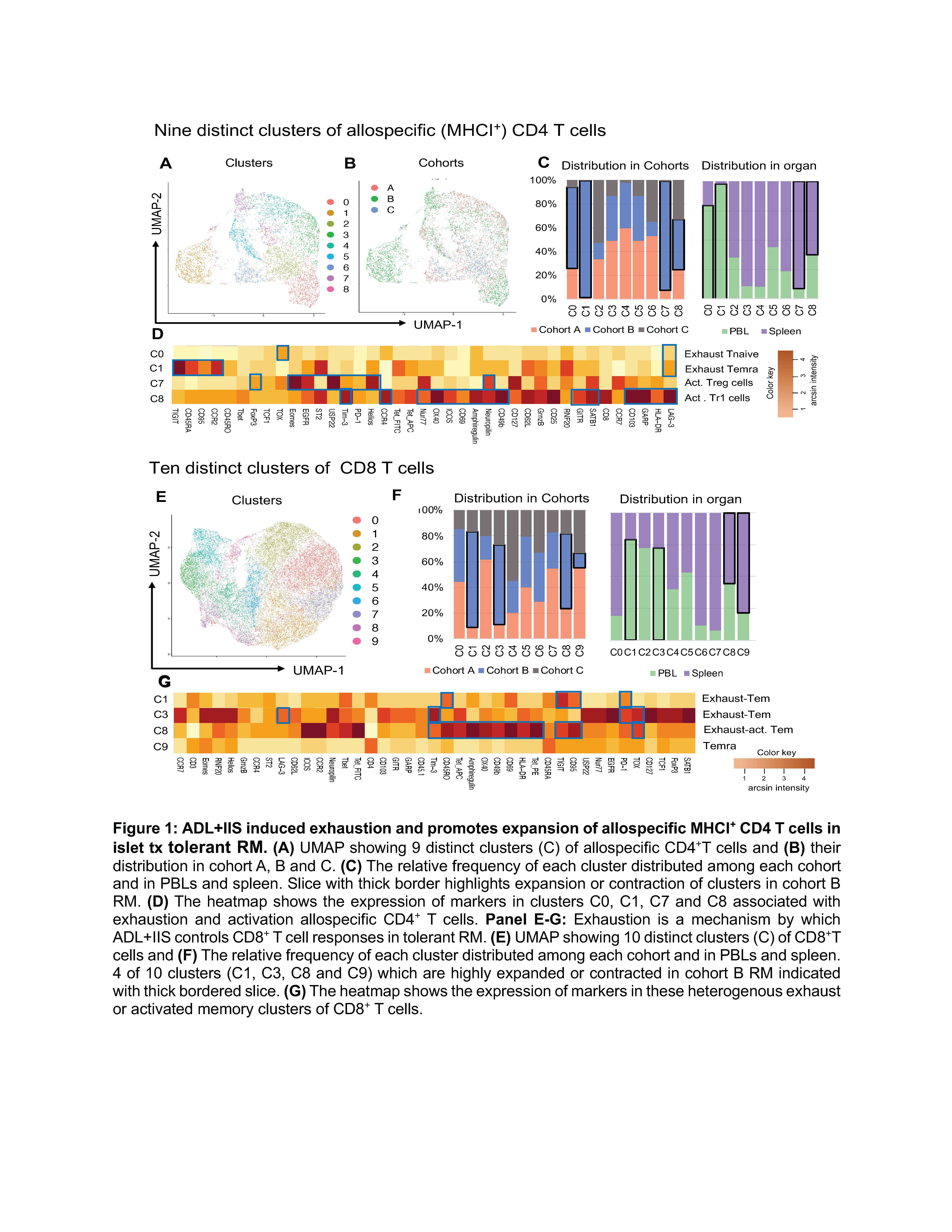
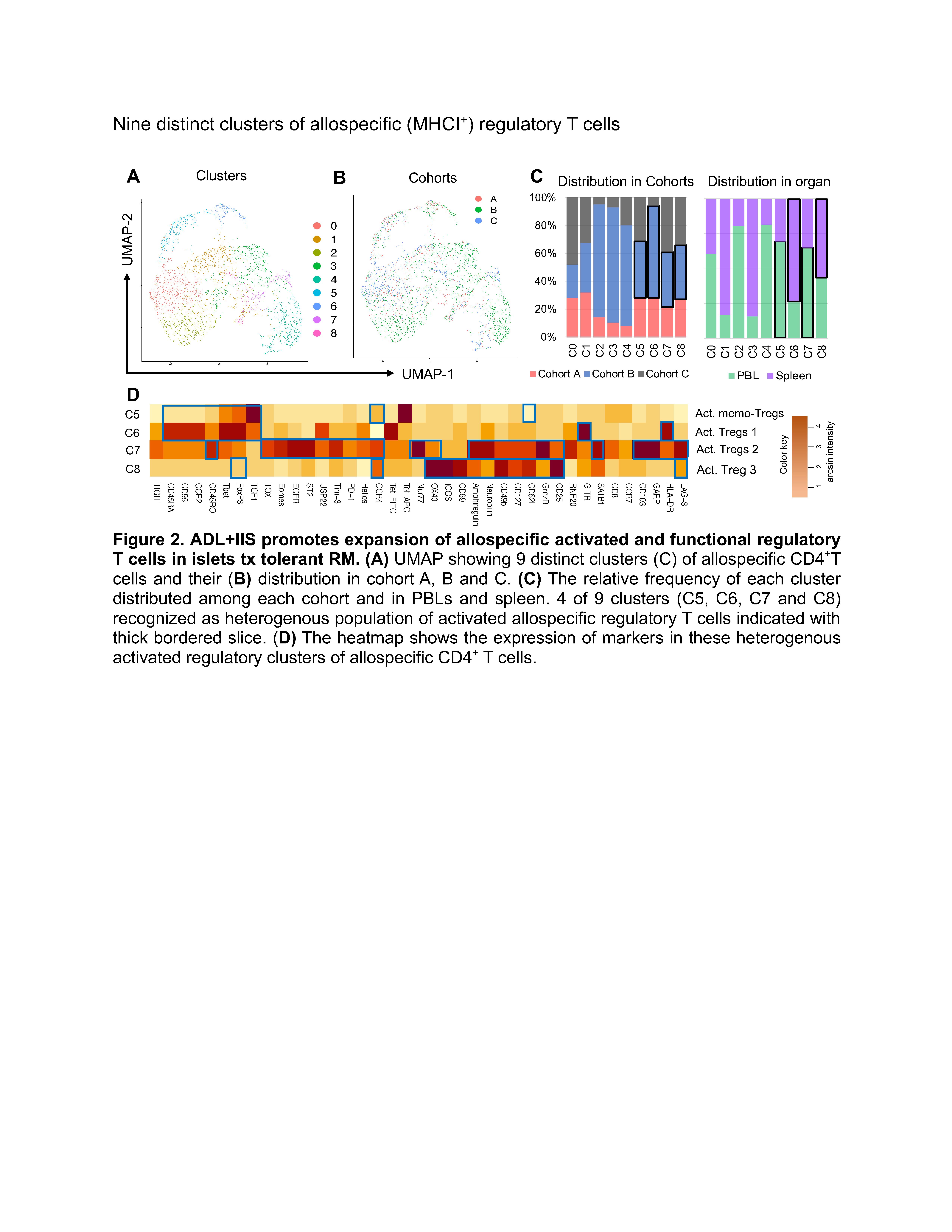
Dissecting the phenotype of allospecific CD4+ T cell in nonhuman primates with long-term tolerance of islet allograft display distinct exhaust and regulatory immune signatures
AMAR SINGH1, Adam Harman1, Anna Tran1, Ravi Masuria1, Anders Matson1, Parthasarathy Rangarajan1, Naoya Sato1, Sarah Vadnais1, Melanie L. Graham1, Sabrinathan Ramachandran1, Bernhard J. Hering1.
1Surgey, University of Minnesota, Minneapolis, MN, United States
Background: For islet cell replacement strategies to be leveraged to their full potential in diabetes care, rejection of transplanted human primary or stem cell-derived islets must be prevented without maintenance immunosuppression. Two peritransplant infusions of apoptotic donor leukocytes (ADLs) under induction immunosuppression (IIS) safely induced long-term tolerance via depletion of alloreactive T cells and expansion of several regulatory cell subsets. A deeper understanding of the immune mechanisms and signatures associated with tolerance induced by the ADL+IIS regimen improves the likelihood of successful clinical translation in islet cell transplantation.
Methods: We employed a 45-parameter CyTOF panel, which spans cell surface and intracellular antigen, validated for use in nonhuman primates, to analyze the identity, abundance, and phenotypic profile of circulatory and splenic T cell subsets in rhesus macaques undergoing islet transplantation by cohort: A (1 DRB-matched with IIS only), B (1 DRB-matched with ADLs+IIS), and C (complete MHC mismatch with ADLs+IIS), each with an n=3. To track allospecific CD4+ T cells, we incorporated MHC class II tetramers that were loaded with mismatched donor MHC class I peptides into the CyTOF panel. The bioinformatics analysis was by unsupervised UMAP clustering to investigate the heterogeneity and functional profiles of T cell subsets.
Results: Our analysis demonstrated the ADL+IIS protocol induced exhaustion in allospecific CD4+ T cells. We identified the presence of 9 distinct clusters of allospecific MHC class I+ CD4+ T cells, among these clusters, 4 were T cells expressing markers associated with exhaustion (C0, C1, C3, and C4). The clusters C0 and C1 (abundant in blood) were expanded in cohort C, while clusters C3 and C4 (abundant in spleen) showed comparable frequencies across cohorts. Next, we examined the regulatory heterogeneity of MHCI+ regulatory T cells (Tregs) and identified 9 distinct clusters, with 4 of 9 clusters recognized as heterogenous subpopulation of activated allospecific regulatory T cells, either showing the phenotype of activated FoxP3+ Tres (C5, C6, and C7) or LAG-3+ CD49bhi FoxP3low Tregs (C8). Among these regulatory clusters, C5 and C7 dominated in the peripheral circulation while C6 and C8 showed high abundance in the spleen compartment.
Conclusions: Our preclinical data in nonhuman primates suggests that the ADLs+IIS regimen in 1 DRB-matched islet transplant recipients promotes both exhaustion of several T cell subsets and activation of distinct Treg and Tr1 regulatory cell subsets. How the interaction of these allo-specific T cell subsets across multiple compartments mediates and potentiates tolerance remains to be determined.