Giuseppe Pettinato, United States
Instructor in Medicine
Medicine
Beth Israel Deaconess Medical Center, Harvard Medical School
Biophysical properties of hiPSC-derived pancreatic organoids
D. Andrea Cristaldi1, Azzurra Sargenti1, Chiara Peres2,3, Daniele Gazzola1, Simone Pasqua1, Simone Bonetti4, Wayne J Hawthorne5, Spartaco Santi2,3, Giuseppe Pettinato6.
1Cell Dynamics iSRL, Bologna, Italy; 2Unit of Bologna, Consiglio Nazionale delle Ricerche (CNR) Institute of Molecular Genetics “Luigi Luca Cavalli-Sforza”, Bologna, Italy; 3Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Istituto Ortopedico Rizzoli, Bologna, Italy; 4CNR-ISMIN, Institute for Nanostructured Materials, Bologna, Italy; 5Department of Surgery, School of Medical Science, Westmead Hospital, University of Sydney, Westmead, NSW, Australia; 6Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Introduction: Differentiated pancreatic organoids represent an important tool for both in vitro studies and potential cell replacement therapy for the treatment of diabetes. Predicting the optimal functionality of a pancreatic organoid using a non-invasive technology will allow for a pre-selection of the best-fit organoids to be used in laboratory, pre-clinical, and clinical research. The unique biophysical properties of hiPSC-derived pancreatic organoids mean they can be used for predictive screening when quantified against benchmark methodologies that verify their physiological function. We have developed organoids for such use and here we demonstrate their utility.
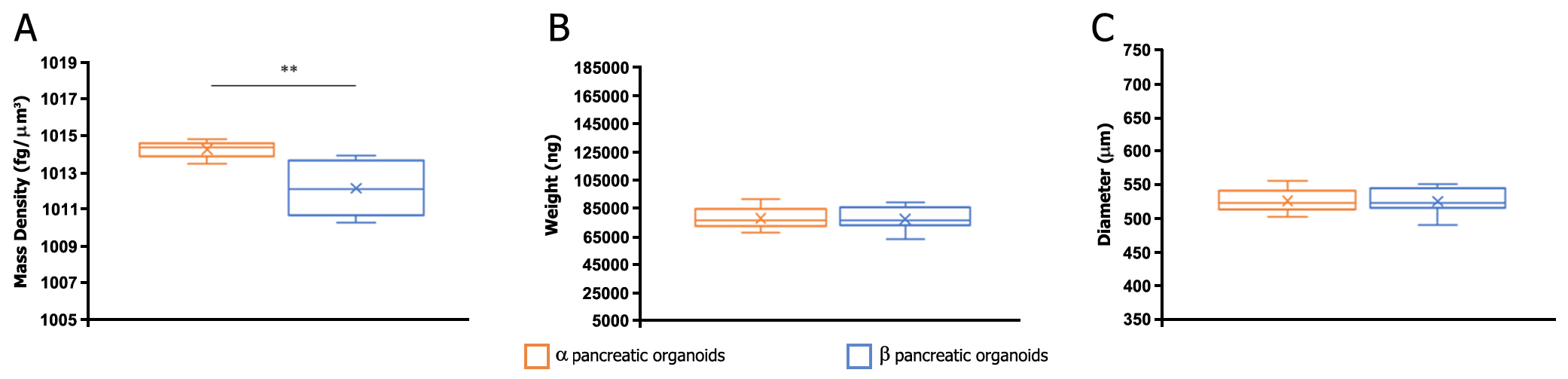
Method: Biophysical characterization was performed using a physical cytometer that allows for simultaneous measurement of mass density, weight, and size of the pancreatic organoids using a label-free technique combined with specific sorting. Two different differentiation protocols for hiPSC-derived pancreatic organoids (named α and β) were applied and initial results obtained with the physical cytometer were compared with the classical biomolecular methodologies for pancreatic function, such as analysis of pancreatic gene markers, and protein expression (data not shown). Organoids were then sorted by size for a fine sample standardization to better highlight biophysical differences. This allowed us to standardize samples derived from both protocols to undertake and improve their comparative analysis.
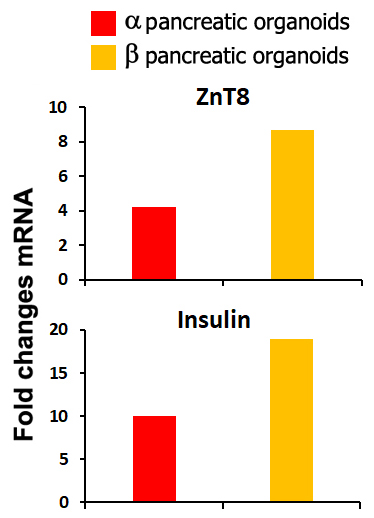
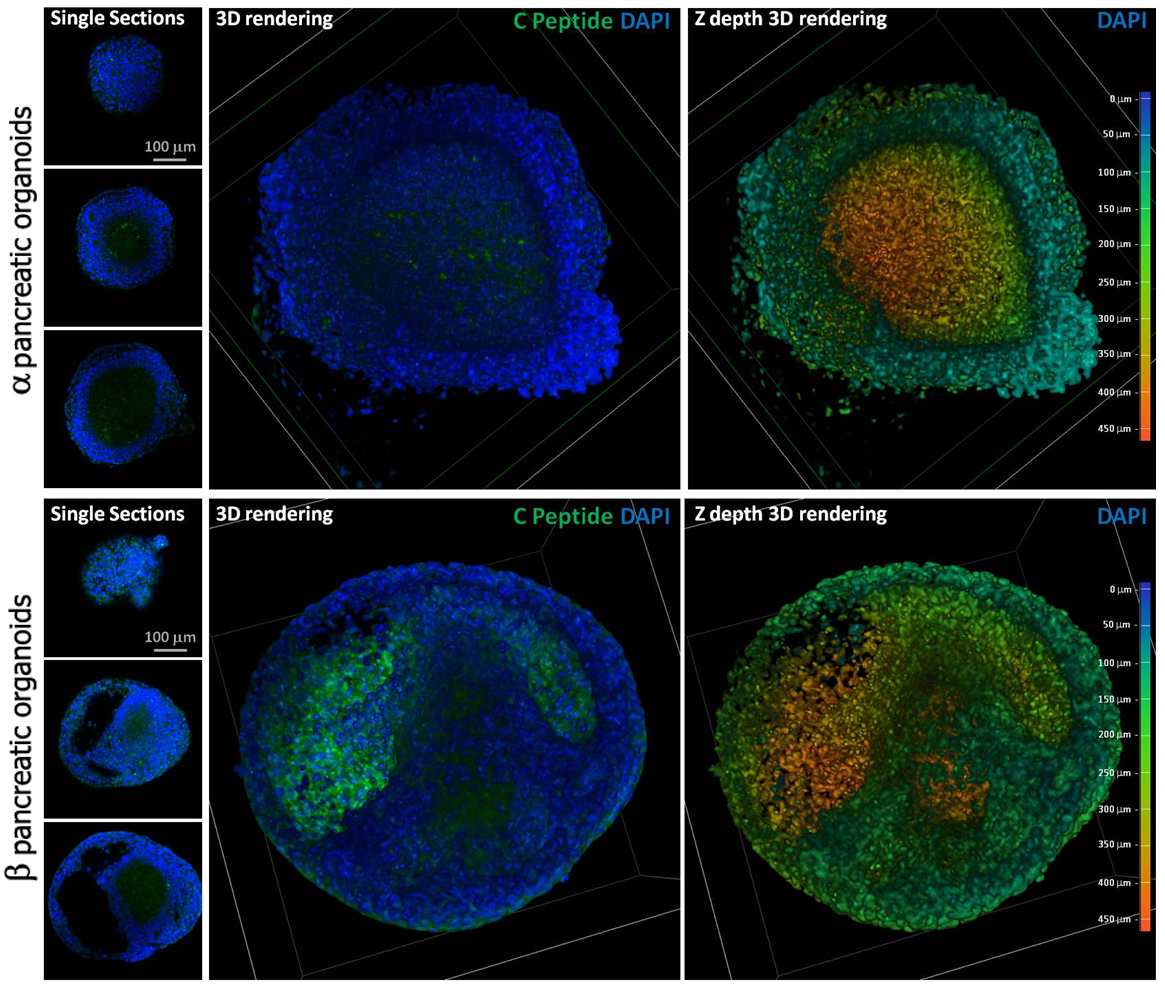
Results: Variations in mass density, obtained on comparable-size organoids, demonstrated, tush correlations between biophysical and functional properties that allowed for direct comparison of biophysical characteristics and active pancreatic function (figure 1) showing a correlation between increased insulin and ZnT8 genes expression in the protocol β (figure 2) with an increased positivity for C-Peptide, as highlighted by immunofluorescence (figure 3). Repeated blinded analysis and measurements of the biophysical characteristics of these organoids, associated with their biomolecular properties, demonstrated that it was possible to select functional organoids based only on their biophysical identity.
Conclusion: These results demonstrate that differentiated pancreatic organoids can be readily pre-screened and monitored using non-invasive techniques, which preserves the integrity of the organoids allowing for their future use, both in vitro and in vivo. This technology can also be used for other types of organoids, either healthy or diseased, such as cancer organoids, allowing for predictive non-invasive screening and clear capacity for comparison in a whole series of disease states.
References:
[1] Cristaldi DA, Sargenti A, Bonetti S, Musmeci F, Delprete C, Bacchi F, Pasqua S, Cavallo C, Bonsi L, Alviano F, Gazzola D, Santi S. A Reliable Flow-Based Method for the Accurate Measure of Mass Density, Size and Weight of Live 3D Tumor Spheroids. Micromachines. 2020 Apr 28;11(5):465.
[2] Pettinato G., Fisher R.A., Perelman L.T. Development of Fully Differentiated Pancreatic Organoids from hiPSC For Type 1 Diabetes Cell Therapy. Transplantation. 104 (S3), S5-S6
[3] Sargenti A, Musmeci F, Cavallo C, Mazzeschi M, Bonetti S, Pasqua S, Bacchi F, Filardo G, Gazzola D, Lauriola M, Santi S. A new method for the study of biophysical and morphological parameters in 3D cell cultures: Evaluation in LoVo spheroids treated with crizotinib. PLoS One. 2021 Jun 8;16(6):e0252907
[4] Ichihara Y, Utoh R, Yamada M, Shimizu T, Uchigata Y. Size effect of engineered islets prepared using microfabricated wells on islet cell function and arrangement. Heliyon. 2016 Jun 28;2(6):e00129.
[5] Huang HH, Stehno-Bittel L. Differences in insulin biosynthesis pathway between small and large islets do not correspond to insulin secretion. Islets. 2015;7(5):e1129097
Lectures by Giuseppe Pettinato
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 11:35 - 12:35 |
Miscellaneous topics 3 | Biophysical properties of hiPSC-derived pancreatic organoids | Indigo 204 |
