Clinical Biomanufacturing Associate 3
Department of Surgery
University of MN- Schulze Diabetes Institute
Naturally purified islets in total pancreatectomy (TP) and islet autotransplant (IAT) for chronic pancreatitis: a model to evaluate impact of exocrine co-transplant on graft function
David Heller1,2, Siri Larsen1,2, Joshua Wilhelm1,2, Greg Beilman2, Ty Dunn4, Timothy Pruett2, Srinath Chinnakotla2,3, Karthik Ramanathan2, Varvara Kirchner5, Bernhard Hering1,2, Melena Bellin2,3.
1Schulze Diabetes Institute, University of Minnesota, Minneapolis, MN, United States; 2Department of Surgery, University of Minnesota, Minneapolis, MN, United States; 3Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States; 4Department of Surgery, Hospital of the University of Pennsylvania, Philadelphia, PA, United States; 5Department of Surgery, Stanford Medicine, Stanford, CA, United States
Introduction: After pancreas digestion for TPIAT, we occasionally observe highly enriched, “naturally purified” (NP) islets, where exocrine tissue has been eliminated by chronic pancreatitis. These NP islets appear similar to COBE purified (CP) islets. We sought to understand whether these NP islets maintain function, and the impact of transplanted exocrine tissue on graft function.
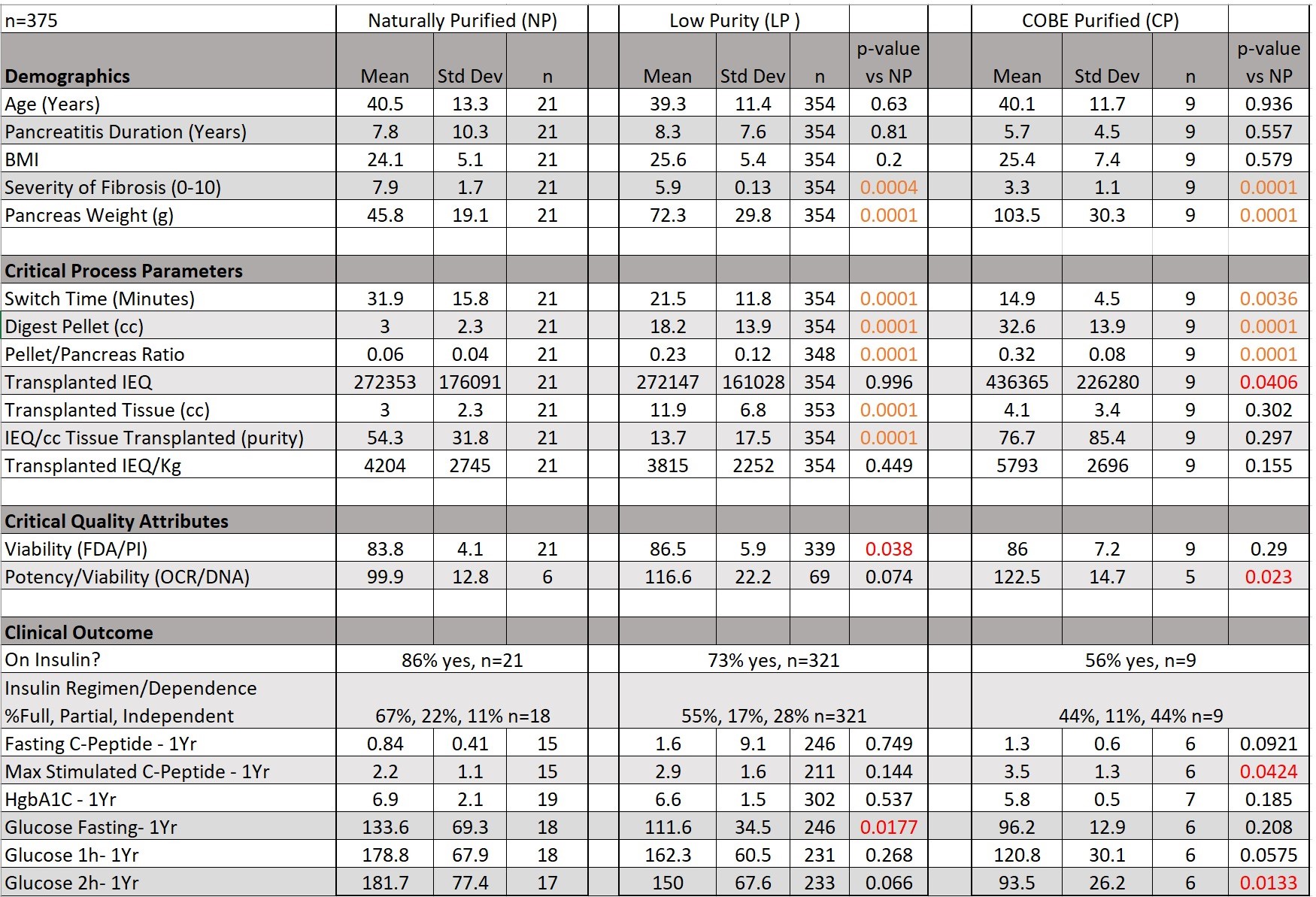
Methods: Patients, age ≥20 years, who received IAT products with volume ratio of ≤ 2:1 exocrine to islets were grouped as NP (n=21) or CP (n=9), and their demographics, isolation, and clinical outcomes compared. The volume of an IEQ was estimated as 5nL or 200,000 islet equivalents (IEQ) per mL. The NP cohort was additionally compared to a low purity ("LP”, > 2:1 exocrine to islets) cohort (n=354). Marginal mass subgroups were defined as those where patients received between 2,500 and 5,000 IEQ/kg of body weight.
Results: Between the NP cohort and the LP and CP groups, fibrosis severity (7.9 vs 5.8 & 3.3), pancreas weight (45.8g vs 72.3 & 103.5), switch time (31.9min vs 21.5 and 14.9), digest pellet (3.0cc vs 18.2 & 32.6), and pellet/pancreas ratio (0.06 vs 0.23 and 0.32) varied significantly. Transplanted tissue volume (2.9cc vs 11.9) and viability (83.8% vs 86.5) were significantly lower in NP vs LP groups. NP patients had significantly higher pre-TPIAT glucose levels at 1 hour (106mg/dL vs 63, p<0.012) during mixed meal tolerance test (MMTT), but not at 2hrs (89mg/dL vs 89, p<0.987) vs CP. NP islet potency (OCR/DNA) was significantly lower than CP (100 nmol/(min · mg DNA) vs 122.5).
One-year post-TPIAT, fasting glucose was higher in NP vs LP (134 mg/dL vs 112), and 2hr-MMTT glucose response was higher in NP vs CP (182 mg/dL vs 93.5). Stimulated c-peptide response was also lower in NP vs CP (2.2 ng/mL vs 3.5). All other 1yr outcomes (basal & max-stim C-pep and glucose, HgbA1C, insulin status) were similar between NP and LP or CP groups. When restricting analysis to marginal mass subgroups, clinical outcomes were similar to those previously stated and retained significant differences. All data is presented in Table 1.
Conclusion: NP islets endure severe, prolonged disease, require extensive enzyme exposure to isolate, but retain endocrine function. Small differences between NP and CP cohorts in meal-stimulated glucose and C-peptide after TPIAT may be due to higher islet mass in the less severely diseased CP group. Data from our cohort suggest there is no clinical detriment from acinar co-transplant, nor any negative effect on islet function from density-gradient purification, as clinical outcomes were similar in naturally purified, COBE-purified, and low purity islet preparations.
References:
[1] Bellin, M; Sutherland, DER; Beilman, G; Hong-McAtee, I; Balamurugan, AN; Hering, BJ; Moran, A. Similar Islet Function in Islet Allotransplant and Autotransplant Recipients, Despite Lower Islet Mass in Autotransplants. Transplantation Vol 91(3), 15 February 2011, pp 367-372
[2] Sutherland, D; Radosevich, DM; Bellin, MD; Hering, Bj; Beilman, Gj ; Dunn, Tb ; Chinnakotla, S; Vickers, SM; Bland, B; Balamurugan, AN; Freeman, Ml; Pruett, T. Total Pancreatectomy and Islet Autotransplantation for Chronic Pancreatitis. Journal Of The American College Of Surgeons, 2012 Apr, Vol.214(4), pp.409-424
