Doctoral Researcher
Chair for Molecular Animal Breeding and Biotechnology, and Center for Innovative Medical Models (
Ludwig-Maximilians-University Munich
[68Ga]Ga-DOTA-Exendin-4 PET/CT detects increased liver signal of transplanted neonatal islet cell clusters in diabetic pig recipients
Nicol Gloddek1,2, Johanna Pilz1,2, Felix Lindheimer3, Magdalena Lindner3, Peter Bartenstein3, Sibylle Ziegler3, Daniel Puhr-Westerheide4, Eckhard Wolf1,2, Elisabeth Kemter1,2.
1Chair for Molecular Animal Breeding and Biotechnology, GeneCenter - Department of Veterinary Science, LMU Munich, Munich, Germany; 2Center for Innovative Medical Models (CiMM), Department of Veterinary Sciences, LMU Munich, Munich, Germany; 3Department of Nuclear Medicine, University Hospital, LMU Munich, Munich, Germany; 4Department of Radiology, University Hospital, LMU Munich, Munich, Germany
Introduction: Transplantation of pancreatic islets into the liver is a curative therapy for patients with poorly controlled type 1 diabetes. So far, there is no common non-invasive method for longitudinal monitoring of the transplanted viable islet cell mass. Exendin-4 as a specific ligand of the glucagon-like peptide 1 receptor (GLP1R), which is localized at high levels on beta cells, might be useful as radionuclide tracer to monitor the viable beta-cell graft mass in the liver by PET/CT in vivo.
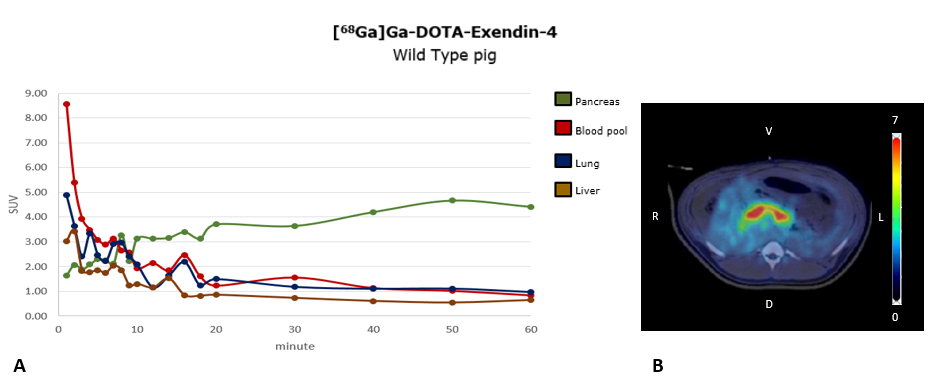
Methods: We first established imaging of the pancreatic beta-cell mass of pigs by [68Ga]Ga-DOTA-Exendin-4 PET/CT, including a dynamic PET scan (60 min) with focus on the pancreas. INSC94Y transgenic diabetic pigs (1) (n = 4; age = 3-4.5 months) received 3,300 - 9,700 IEQ wild-type neonatal pig islets (NPIs) per kg body weight by minimal invasive intraportal transplantation. NPIs were infused in the portal system preferentially of the left liver lobe, that right liver lobe could serve as imaging negative control. NPI recipient pigs got a glucocorticoid-free immunosuppression regime. [68Ga]Ga -DOTA-Exendin-4 PET/CT was performed once prior to islet-cell transplantation, and up to 3-times in the post-transplant period (10-15 weeks). For imaging of the NPI graft mass in the liver, [68Ga]Ga-DOTA-Exendin-4 was injected intravenously (effectively injected activity: 19.9-82.8 MBq; amount of peptide: 0.02 ± 0.005 µg/kg), followed by a dynamic PET/CT (60 min) of the liver region. The activity was analysed 60 min p.i. in the whole liver. Finally, histological analysis of systematically sampled liver tissue was performed to identify graft localization.
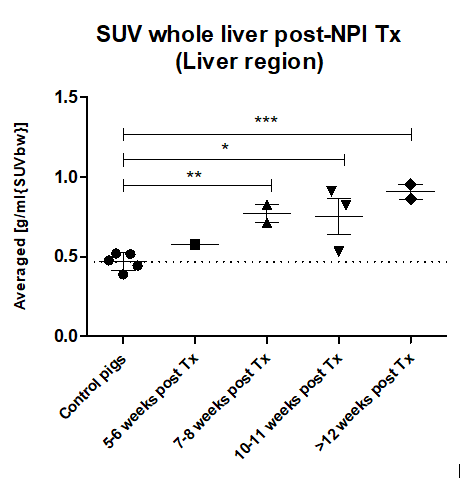
Results: The proof-of-concept [68Ga]Ga -DOTA-Exendin-4 PET/CT imaging study showed a specific signal in pig pancreas (Figure 1), that was significantly reduced in a blocking study with cold Exendin-4. In livers of NPI recipient pigs which reached a normoglycemic status (<130 mg/dl Glc, 3 of 4 pigs), the standard uptake value (SUV) of the liver significantly increased compared to the control values (61% at 7-8 weeks and 94% after 12 weeks; Figure 2). The increased liver signal was multifocal with clusters of individual hotspots, matching the histological distribution pattern of islet in the liver, which contained mainly insulin positive beta cells. No signs of immune rejection were observed in the graft recipient animals reaching normoglycemia.
Conclusion: Viable beta-cell mass after intraportal islet allotransplantation can be detected in a diabetic large animal model by using [68Ga]Ga -DOTA-Exendin-4 in PET/CT. As changes in the viable graft mass over time could be visualized, this new diagnostic tool should also be suitable for longitudinal monitoring of intraportal islet transplants in humans.
The study was supported by the Deutsche Forschungsgemeinschaft (TRR127) and the European Union's Horizon 2020 research and innovation program under grant agreement No. 760986 (iNanoBIT)..
References:
[1] Renner, S. et al. (2013). Permanent neonatal diabetes in INSC94Y transgenic pigs. Diabetes, 62(5), 1505-1511
Lectures by Nicol Gloddek
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 16:00 - 17:30 |
Enabling technologies | [68Ga]Ga-DOTA-Exendin-4 PET/CT detects increased liver signal of transplanted neonatal islet cell clusters in diabetic pig recipients | Indigo 204 |
