Massimo Mangiola, United States
Clinical Associate Professor
Transplant Institute
NYU Langone Health
Anti-pig antibodies in human immunoglobulin preparations
Massimo Mangiola1, Jeffrey Stern1, Jacqueline Kim1, Amy S Dandro2, Karen Khalil1, Sapna A Mehta1, Tyler Lewis1, David Ayares2, Adam Griesemer1, Robert A Montgomery1.
1NYU Langone Transplant Institute, New York, NY, United States; 2Revivicor, Inc., Blacksburg, VA, United States
Introduction: The presence of anti-pig antibodies in human immunoglobulin preparations poses a potential challenge to clinical xenotransplantation with porcine donors.
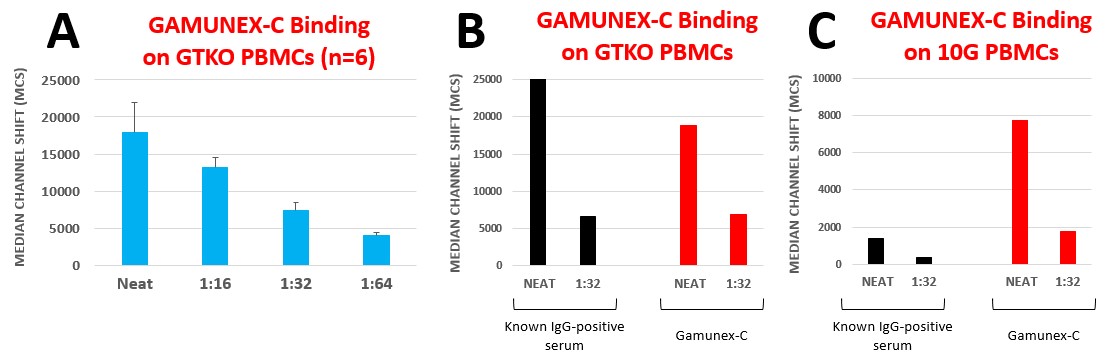
Methods: GTKO and 10GE pigs abrogate hyperacute rejection of porcine xenografts. However, antibodies to other pig epitopes are commonly present in human sera. Human intravenous immunoglobulin (IVIg) preparations pool thousands of units of human plasma that may contain a large number of anti-pig antibodies, which may harm xenografts by passive transfer when administered to xenotransplant recipients. To validate this hypothesis, we tested a Gamunex-C preparation against lymphocytes from GTKO and 10GE pigs. IVIg was diluted to a concentration of 50 mg/ml and binding of anti-pig IgG antibodies to pig lymphocytes was tested using flow cytometry. Gamunex-C was used both undiluted and serially diluted up to 1:64, then tested on lymphocytes from 6 GTKO pigs and one 10GE pig.
Results: As expected, Gamunex-C expresses anti-pig IgG antibodies and reacts to genetically-modified pig lymphocytes even in the absence of the 3 most common xenoantigens. When tested against 6 different GTKO pigs, no significant differences between animals were found (Fig 1A). When compared to a serum known to have strong IgG reactivity against GTKO lymphocytes, Gamunex-C performed similarly (Fig 1B). However, distinct from the control serum, when the preparation was tested against 10GE lymphocytes, IgG binding reactivity was still significant at 1:32. Based on our in vivo experience, this reactivity strength identifies antibodies that lead to complement activation.
Conclusions: Our study demonstrates that IVIg preparations contain significant amounts of anti-pig IgGs and may contribute to xenograft rejection by activation of the complement cascade. Therefore, IVIg treatment of antibody-mediated rejection in xenotransplantation should be avoided. Similar considerations apply to any blood products that contain human plasma. Additional studies on FFP, RBC supernatants, platelet supernatants, and cryoprecipitate are ongoing to better understand the risk of passive transfer of anti-pig IgG antibodies.
Supported by Lung Biotechnology, a wholly owned subsidiary of United Therapeutics..
Lectures by Massimo Mangiola
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sun-29 10:00 - 11:30 |
Monitoring pre-clinical and clinical application of xenotransplantation | Anti-pig Antibodies in Human Immunoglobulin Preparations | Indigo H |
|
Sat-28 12:50 - 13:50 |
eGenesis Symposium on Allografts and Xenografts: Friends or Foes? | Debate #1: Sensitized patients are at high risk for xenotransplantation - AGAINST | Indigo BC |
