Alexander J Sagar, United Kingdom
Clinical Research Fellow
Nuffield Department of Surgical Sciences
University of Oxford
48-hour normothermic xenoperfusion of genetically engineered porcine livers with whole human blood
Alexander Sagar1, Kathryn Stiede2, Kristen Getchell2, Leanne Lanieri2, Hussain Abbas1, John Fallon1, Mohamed Elzawahry1, Paula Hamilton2, Carson Sutter2, Amanda Marett2, Michael Etter2, Cassandra Miller2, Kayla Janke2, Raffety Wyatt2, Raquel Castro2, Chris Morris3, Dan Fower3, Lydia Lamriben2, Kelly Cederquist4, Federick Vyas4, Peter Abt4, Susan Low2, Peter Friend1,3.
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom; 2eGenesis Inc, Cambridge, MA, United States; 3OrganOx, Oxford, United Kingdom; 4Division of Transplantation, Hospital of the University of Pennsylvania, Philadelphia, PA, United States
Introduction: Normothermic machine perfusion (NMP) has been developed as a technique of organ preservation, in which the liver is perfused with blood, nutrients, and oxygen at body-temperature. NMP may be used as a platform to assess the haematological, immunological, and functional implications of xenoperfusion and safely evaluate any major obstacles to hepatic xenotransplantation in an ex vivo setting.
This technique also has direct clinical implication: by demonstrating maintenance of physiological activity across the full range of hepatic function, extracorporeal liver perfusion has the potential to provide temporary support to patients with acute liver failure.
Methods: Livers were procured from anaesthetised genetically modified porcine donors that had triple glycan knockout and hemizygous insertion of human coagulation, complement and immunological genes (EGEN-5734 or EGEN-5784). In addition, EGEN-5784 donor livers had functional inactivation of porcine endogenous retrovirus (PERV) pol gene.
Following backbench preparation, the livers underwent NMP on the OrganOx metra using pooled whole human blood for 48 hours (n=3). Parenchymal appearance, haemodynamic parameters, metabolic activity, bile production and full blood counts were monitored.
Results: All three livers demonstrated stable and physiological perfusion characteristics throughout the 48-hour period.
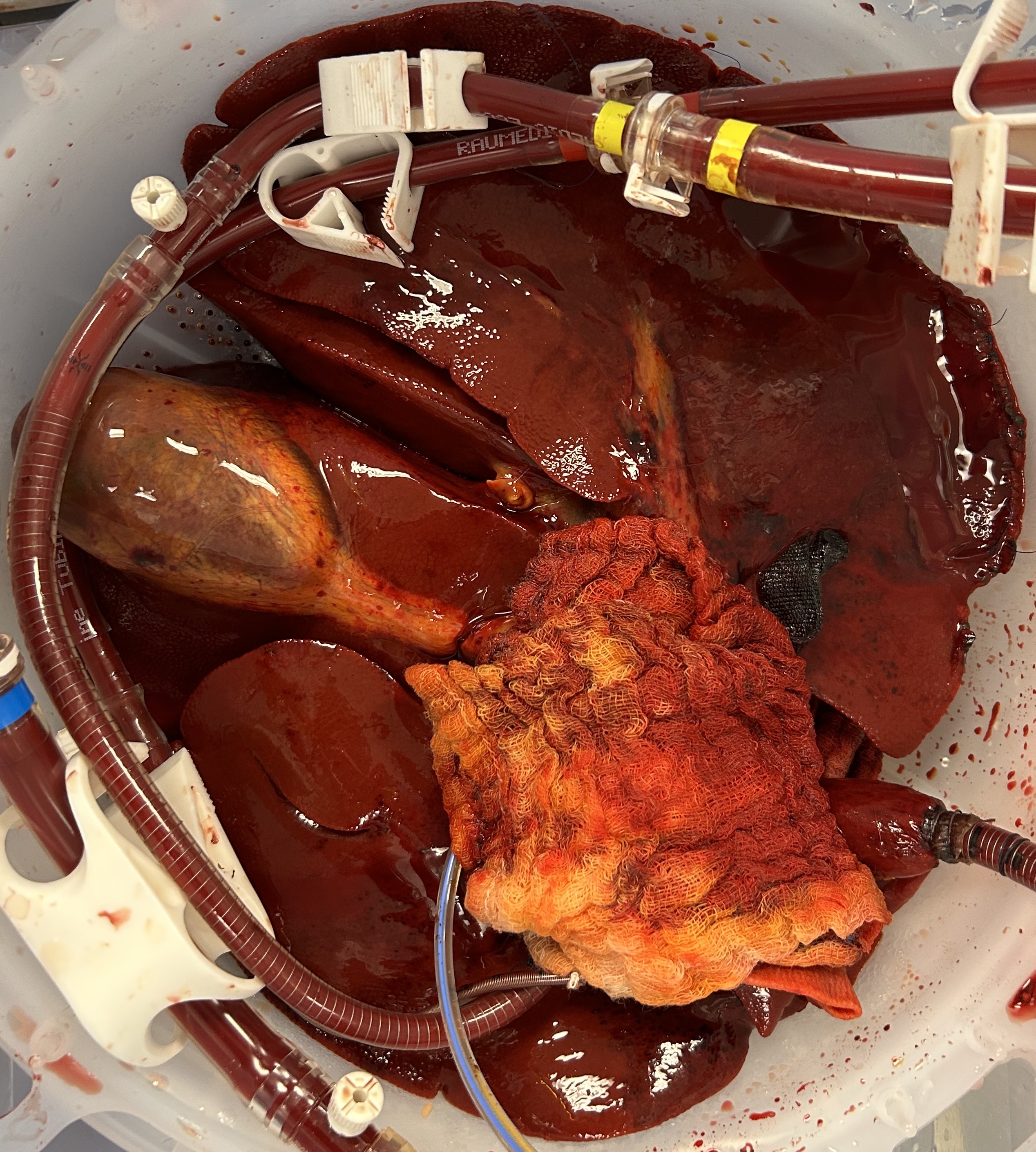
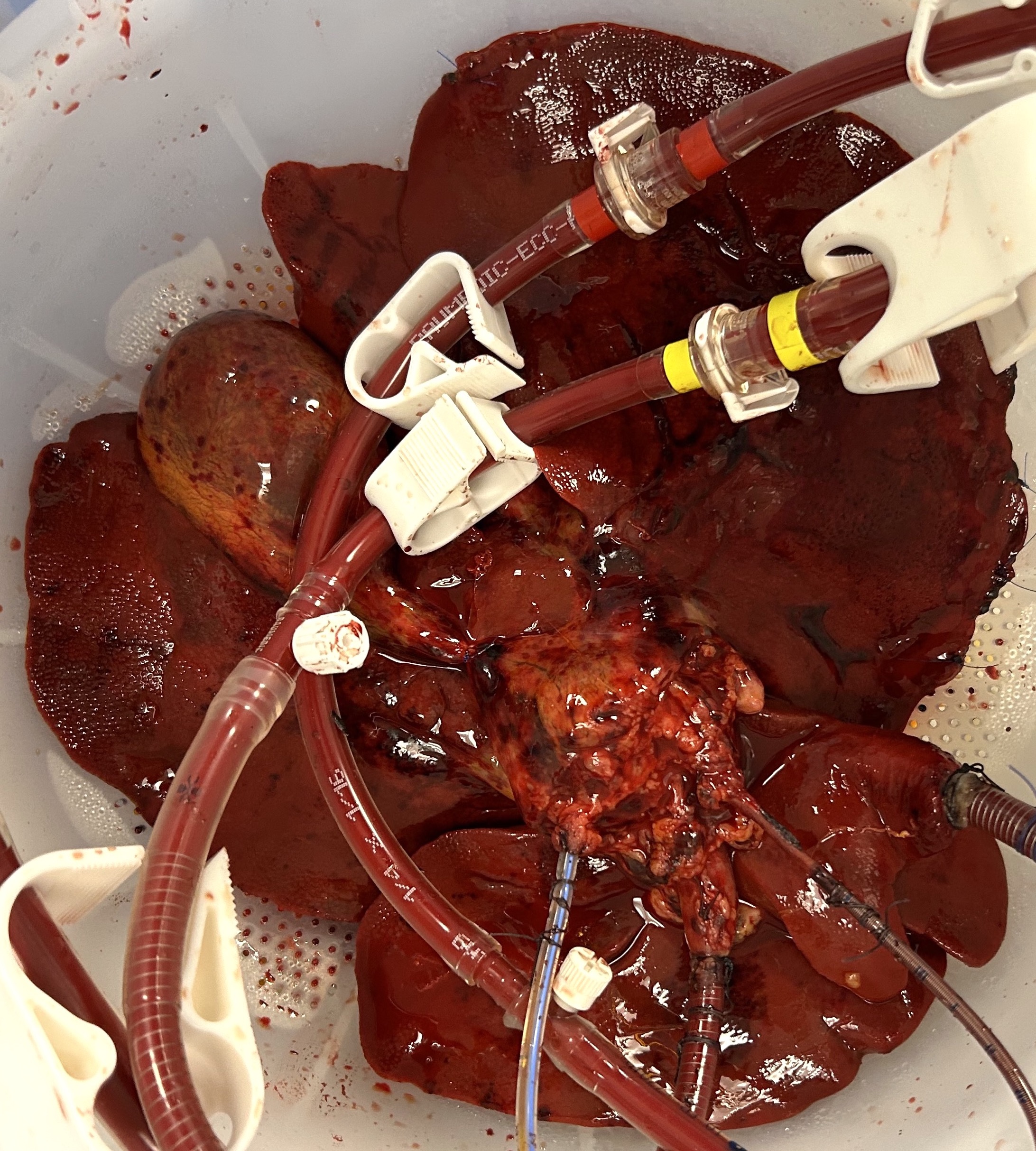
The macroscopic appearance at 48 hours demonstrated homogenous parenchymal perfusion (figures 1 and 2), with mean 48-hour flow rates of 320 (200 – 480) mL/min via the hepatic artery and 880 (780 – 970) mL/min via the portal vein.
Metabolic function was maintained, with consistent glucose consumption throughout the 48-hour period and a mean final lactate of 1.89 (1.15 – 2.74) mmol/L. Excretory function was similarly preserved with a mean bile production rate of 12 (5 – 23) mL/hr over 48 hours.
The mean 48-hour haemoglobin level was 12.5 (10.2 – 15.1) g/dL with a median of 1 (0-1) unit whole blood supplementation during the perfusion period.
Conclusion: This study demonstrates successful perfusion of genetically engineered EGEN-5734 and EGEN-5784 porcine livers with whole human blood over 48 hours, without immunosuppressive drugs. Haemodynamic and functional parameters were preserved, and there was no evidence of significant haemophagocytosis.
This NMP model provides valuable evidence that confirms the compatibility of genetically engineered porcine organs with human blood and may lead to the first clinical use of xenogeneic livers in the context of short-term extracorporeal liver support for patients with acute liver failure.
Lectures by Alexander J Sagar
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 16:00 - 17:30 |
Enabling technologies | 48-hour normothermic xenoperfusion of genetically engineered porcine livers with whole human blood | Indigo 204 |
