10-year follow-up of type 1 diabetic patients following an encapsulated porcine islet xenotransplantation without immunosuppression
Shinichi Matsumoto1,2, Adrian Abalovich3, Shaun Wynyard1, Mariana E Carulla3, David Abalovich3.
1Diatranz Otsuka Ltd, Auckland, New Zealand; 2Otsuka Pharmaceutical Factory Inc, Naruto, Japan; 3Hospital Eva Peron de San Martin, Buenos Aires, Argentina
Background: Intensive insulin therapy is the standard therapy for type 1 diabetes, however, hypoglycemia is a critical limiting factor. Allogeneic islet transplantation can effectively prevent severe hypoglycemic events and provide excellent blood glucose control (1). However, donor shortage and necessity of immunosuppression are two major issues to expand allogeneic islet transplantation (2). To overcome the two issues, we performed clinical encapsulated neonatal porcine islet transplantation without immunosuppression for the treatment of type 1 diabetes, and demonstrated the efficacy and safety (3-7). In this study, we follow-up the patients and analyze the patient opinions for encapsulated islet xenotransplantation without immunosuppression 10 years after transplantation.
Methods: Twenty one type 1 diabetic patients received microencapsulated porcine islet xenotransplantation in Argentina were enrolled. Seven patients were enrolled in efficacy and safety study and 14 patients were enrolled in safety studies. Opinions of all 21 patients related to the current and pre-transplant status of diabetes control, blood glucose levels, severe hypoglycemia and hyperglycemia required hospitalization were analyzed. In addition, their opinions related to islet xenotransplantation were assessed.
Results: After transplantation all patients significantly improved HbA1c and 5 out of 7 patients significantly reduced daily insulin dose (Figure)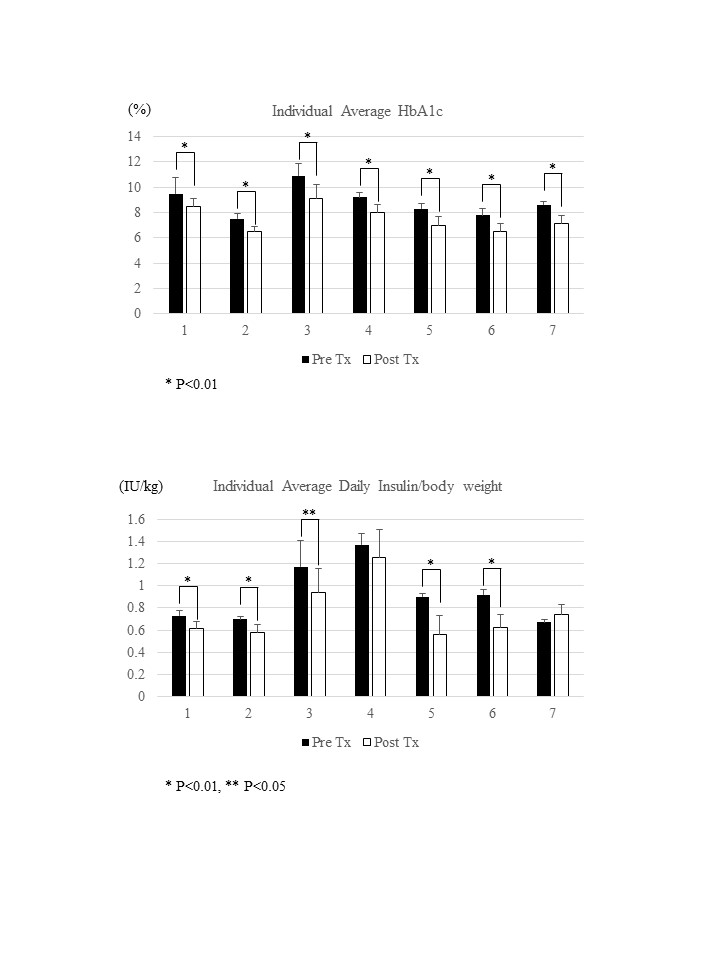 .
.
At the time of this survey, average HbA1c of seven patients were significantly lower compared to pre-transplantation (8.5 ± 0.9 (%) at pre-transplant and 7.4 ± 0.5 (%) at the survey, p<0.05) and average insulin dose were also lower compared to pre-transplantation (0.95 ± 0.32 (IU/kg) at pre-transplant and 0.73 ± 0.27 (IU) at the survey).
Based on patient opinions, the majority of patients improved diabetes control (71%), blood glucose levels (76%), and reduced severe hypoglycemia (86%) and hyperglycemia required hospitalization (76%). No patients deteriorated in all of the categories when compared with pre-transplantation. No patients had cancer, psychological problem and one patient had a serious adverse event. The majority of patients wanted to recommend this treatment to other patients (76%) and receive booster transplantation (85.7%).
Conclusions: The majority of patients had positive opinions related to the encapsulated porcine islet xenotransplantation without immunosuppression 10 years after transplantation.
[1] Hering BJ, Clarke WR, Bridges NC, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016; 39: 1230-1240.
[2] Matsumoto S, Shimoda M. Current situation of clinical islet transplantation from allogeneic toward xenogeneic. J Diabetes 2020; 12:733-741.
[3] Matsumoto S, Tan P, Baker J, et al. Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant Proc 2014;46: 1992-1995.
[4] Wynyard S, Nathu D, Garkavenko O, Denner J, Elliott R. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation 2014; 21: 309-323.
[5] Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical benefit of islet xenotransplantation for the treatment of type 1 diabetes. EBioMedicine 2016; 12: 255-262.
[6] Morozov VA, Wynyard S, Matsumoto S, Abalovich A, Denner J, Elliott R. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res 2017; 227: 34-40.
[7] Matsumoto S, Wynyard S, Giovannangelo et al. Long-term follow-up for the microbiological safety of clinical microencapsulated neonatal porcine islet transplantation. Xenotransplantation 2020; 27: e12631