Calcium sensor reporter pig model for assessment of beta-cell function
Sarah Grimus1,2, Christian M. Cohrs3,4, Mayuko Kurome1,2, Valeri Zakhartchenko1,2, Barbara Kessler1,2, Stephan Speier3,4, Eckhard Wolf1,2, Elisabeth Kemter1,2.
1Chair for Molecular Animal Breeding and Biotechnology, GeneCenter - Department of Veterinary Science, LMU Munich, Munich, Germany; 2Center for Innovative Medical Models (CiMM), Department of Veterinary Science, LMU Munich, Munich, Germany; 3Institute of Physiology, Faculty of Medicine, TU Dresden, Dresden, Germany; 4PLID of the Helmholtz Zentrum Munich at the University Hospital and Faculty of Medicine, TU Dresden, Dresden, Germany
Introduction: Xenotransplantation of porcine islets is a promising approach for the treatment of patients with insulin-deficient diabetes mellitus. The optimization of strategies to improve islet engraftment and long-term survival is an active field of preclinical research. Ca2+ influx into beta cells is an important driver of insulin secretion. Therefore, monitoring of Ca2+ kinetics in beta cells provides an important readout for the maturation of neonatal porcine islets (NPIs) in vitro and of NPI grafts in vivo.
Methods: The coding sequence for the ultra-sensitive protein calcium sensor GCaMP6, codon optimized for expression in mammalian cells, was put under the control of a ubiquitous CAG promoter. Porcine primary kidney cells were nucleofected with the CAG-GCaMP6 expression construct and after 5 days cells showing GFP fluorescence due to spontaneous Ca2+ transients were isolated by flow cytometry. Somatic cell nuclear transfer of these cells resulted in one pregnancy and birth of 2 male piglets, thereof 1 stillborn. Validation of transgene expression was first performed on a tail sample of the living founder pig, and later on tissue samples of his offspring. Pancreas tissue of his neonatal offspring was used for NPI isolation and NPI transplantation into the anterior chamber of the eye (ACE) in mice for NPI graft maturation. Ca2+ kinetics was assessed during live cell imaging on NPIs and on the explanted graft 8 weeks after transplantation upon a glucose-stimulated insulin secretion (GSIS) test.
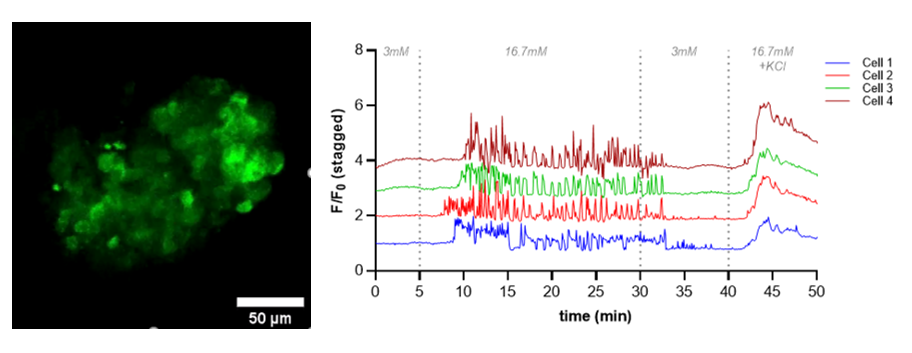
Results: Strong ubiquitous expression of the CAG-GCaMP6 transgene both in the tail sample of the founder pig as well as in all tissue samples such as pancreas was proven by GFP immunohistochemistry. A strong ubiquitous GCaMP6 expression in NPIs was detected. Upon potassium chloride challenge in in vitro GSIS, most cells of NPIs showed highest fluorescence response. However, only few cells exhibited a glucose-induced Ca2+-induced response represented by a wave of increased measured fluorescence intensity in in vitro matured NPIs indicating few contents on mature beta cells in these NPIs. NPIs engrafted in the mouse AEC did not show a Ca2+ reporter response upon glucose challenge, due to inhibitory effect of isoflurane anesthetic on glucose-induces ATP and Ca2+ increase. In contrast, multiple pulsatile Ca2+ reporter responses in numerous cells were measured upon glucose stimulus of AEC NPI grafts ex vivo, with highest response upon final potassium chloride stimulus (Fig. 1). Immunohistochemical staining of the AEC NPI grafts confirmed a high proportion of beta cells in the grafts.
Conclusion: Live cell imaging of Ca2+ kinetics in GCaMP expressing NPIs represents a novel tool to evaluate the beta cell maturation stages both in vitro and in vivo.
The study was supported by the Deutsche Forschungsgemeinschaft (TRR127) and by the German Federal Ministry of Education and Research (BMBF) to the German Centre for Diabetes Research (DZD e.V.) (Grant No. 82DZD00802). .