Initial investigation on the feasibility of porcine red blood cells from genetically modified pigs as a human red blood cell alternative for transfusion
Sangkeun Park1,2, Haneulnari Lee1, Eun Mi Park1, Juhye Roh1, Pul Ip Kang3, Joohyun Shim3, Kimyung Choi3, Hee Jung Kang1,2.
1Department of Laboratory Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea; 2Department of Laboratory Medicine, Hallym University College of Medicine, Chuncheon, Korea; 3Optipharm Inc., Cheongju, Korea
Background: The decline in blood donation rates and ongoing shortages of blood products pose significant challenges for medical societies worldwide. One potential solution is the use of porcine red blood cells (pRBCs) from genetically modified pigs as an alternative to human red blood cells (hRBCs), due to their unlimited supply and ease of infection control. However, adverse immunological reactions, including hemolysis, remain a significant obstacle to their use. This study aimed to evaluate the performance of diverse genetically modified pRBCs in human serum regarding antibody reactivities, complement activation, and human serum-mediated hemolysis.
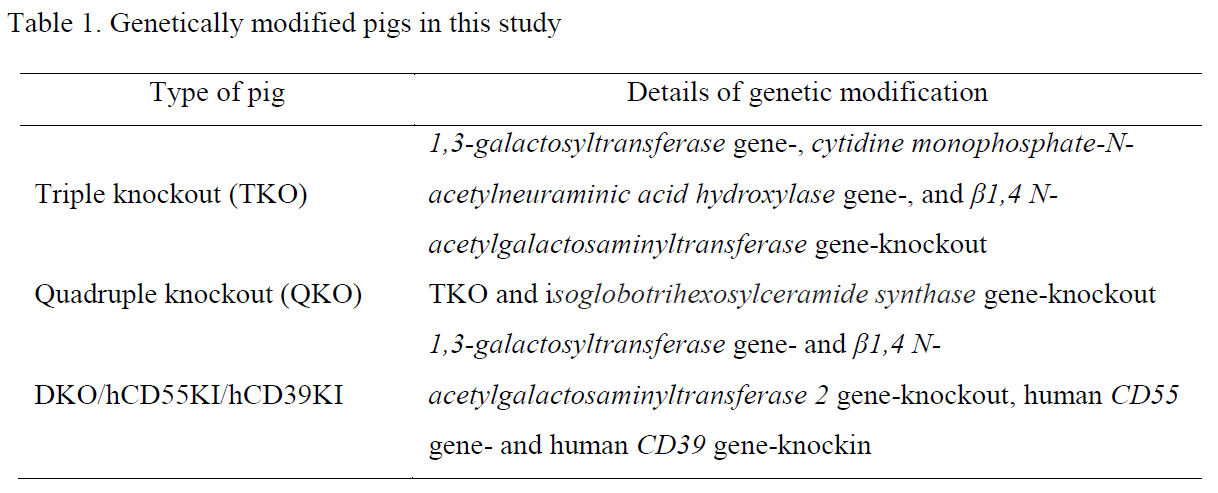
Methods: We commercially acquired human complement-competent serum, complement 7 (C7)-deficient serum, and hRBCs of all ABO blood types. Additionally, we utilized leftover clinical samples from health checkups for further evaluation. The pRBCs were collected from wild-type (WT) and genetically modified pigs (Table 1). The extent of C3 deposition on the RBCs was measured by flow cytometry post-incubation in C7-deficient serum diluted in Ca++-enriched (total complement activity) or Ca++-depleted buffer (only alternative pathway complement activity). Immunoglobulin (Ig)M/IgG antibody binding to the RBCs post-incubation in each ABO type human serum was evaluated by flow cytometer. Human serum-mediated hemolysis of O-type hRBCs and genetically modified pRBCs was also tested.
Results: All three genetic modifications significantly improved the pRBCs compatibility with human serum compared to WT pRBCs. However, the extent of IgM/IgG binding to pRBCs varied individually. Total complement activation on TKO or QKO pRBCs was weaker than that on DKO/hCD55KI/hCD39KI pRBCs, while alternative pathway complement activation did not differ between them. DKO/hCD55KI/hCD39KI pRBCs hemolysis was significantly higher than that of TKO or QKO pRBCs, which were comparable to that of type O hRBCs.
Conclusion: The elimination of porcine carbohydrate antigens in TKO or QKO pigs resulted in a marked enhancement of pRBC compatibility with human serum compared to ABO-incompatible hRBCs and WT pRBCs. The improved compatibility was comparable to that of type O hRBCs. However, DKO/hCD55KI/hCD39KI pRBCs exhibited lower compatibility and higher classical pathway complement activation than TKO pRBCs. These findings provide valuable insights for developing pRBCs as a potential alternative to hRBCs.
This research was financially supported by the Institute of Civil Military Technology Cooperation funded by the Defense Acquisition Program Administration and Ministry of Trade, Industry and Energy of Korean government under grant No. 22-CM-EC-18..