Intraportal transplantation of neonatal porcine islets cures diabetes mellitus of INSC94Y transgenic pigs
Johanna Pilz 1,2, Nicol Gloddek1,2, Daniel Puhr-Westerheide3, Muzzafer Ümütlü3, Eckhard Wolf1,2, Elisabeth Kemter1,2.
1Chair for Molecular Animal Breeding and Biotechnology, GeneCenter - Department of Veterinary Science, LMU Munich, Munich, Germany; 2Center for Innovative Medical Models (CiMM), Department of Veterinary Sciences, LMU Munich, Munich, Germany; 3Department of Radiology, University Hospital, LMU Munich, Munich, Germany
Introduction: Islet transplantation (Tx) is a promising strategy to cure type 1 diabetes. However, a broad clinical application is currently limited by a shortage of islet donors. A promising alternative islet source are pig islets. Development and validation of novel beta-cell replacement therapies are mainly examined in small animal models or in non-human primates (NHP), both associated with several limitations in translating findings to human patients. Therefore, a non-NHP large animal model for islet transplantation using diabetic INSC94Y transgenic pigs was established and used to evaluate the efficacy of intraportal neonatal porcine islet (NPI) transplantation.
Methods: Minimal invasive percutaneous intraportal islet transplantation (PIPIT) was performed to transplant wild-type NPIs (3,300 – 9,700 IEQ/kg body weight) in diabetic INSC94Y transgenic pigs (n = 4) (1). Immunosuppressive regime was adapted from the Edmonton protocol. It consisted of tacrolimus (0.25 mg/kg BW) and mycophenolate mofetil (500 mg) every 12 hours orally, starting one day before transplantation. In addition, the pigs obtained a daily dose of acetylsalicylic acid (100 mg) as anti-platelet treatment. In the observation period, post transplantation fasting blood glucose values were examined on a regular basis. For animal welfare reasons exogenous insulin replacement therapy was performed according to the measured blood glucose values. After a post-transplantation monitoring period between 71 to 103 days, the liver was systematically processed for detailed histological analysis of graft clusters.
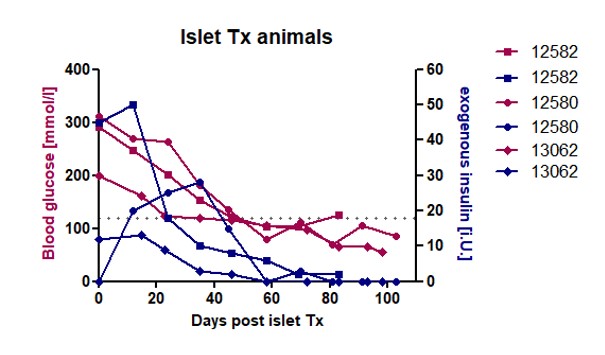
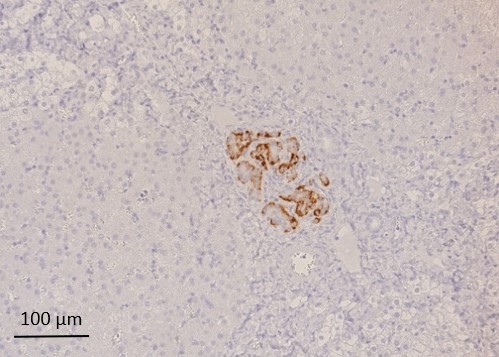
Results: Three of four pigs which had received NPI grafts reached normoglycemia (<130 mg/dl glucose) after 2 months, thereof two without need of exogenous insulin therapy. These animals accepted oral immunosuppressive drug intake constantly and had a continuous decrease of blood glucose concentration, paralleled with reduced need of exogenous insulin therapy (Fig. 1). The fourth animal lowered blood glucose level constantly below 200 mg/dl after NPI transplantation. Histological analysis of graft-bearing livers revealed graft clusters of heterogenous size (19 µm x 44 µm – 250 µm x 453µm) mainly consisting of beta cells (Fig. 2), with a broad distribution over the transplanted liver lobe. In total, 72 paraffin-embedded liver slices per animal were analyzed with immunohistochemistry. On average, 41% of the examined slices contained islet cell clusters, of which Ø 69% were insulin positive.
Conclusion: NPI grafts were able to restore normoglycemia in diabetic recipient pigs. Furthermore, islet Tx lowered or eliminated the need of exogenous insulin treatment. Therefore, this animal model is a novel tool in regenerative medicine to investigate islet cell transplantation settings with a high comparability to human islet cell transplantation.
The study was supported by the Deutsche Forschungsgemeinschaft (TRR127) and the European Union's Horizon 2020 research and innovation program under grant agreement No. 760986 (iNanoBIT). .
[1] (1) Renner, S., et al. (2013). "Permanent Neonatal Diabetes in INSC94Y Transgenic Pigs." Diabetes 62(5): 1505-1511.