No differences in long-term diabetes outcomes with etanercept or alpha-1 antitrypsin treatment in total pancreatectomy and islet autotransplantation: A randomized controlled pilot study
Tasneem Abdel-Karim1, James S Hodges2, Timothy L Pruett3, Karthik V Ramanathan3, Bernhard J Hering3, Ty B Dunn3,4, Varvara A Kirchner3,5, Gregory J Beilman3, Melena D Bellin1,3.
1Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States; 2Department of Biostatistics, University of Minnesota, Minneapolis, MN, United States; 3Department of Surgery, University of Minnesota, Minneapolis, MN, United States; 4Department of Surgery, University of Pennsylvania, Philadelphia, PA, United States; 5Department of Surgery, Stanford University, Palo Alto, CA, United States
Introduction: In total pancreatectomy with islet autotransplant (TP-IAT) for chronic pancreatitis, early islet damage from the instant blood-mediated inflammatory response (IBMIR) compromises diabetes outcomes. We previously reported that in our randomized trial with etanercept or alpha-1-antitrypsin (A1AT) to block IBMIR, etanercept-treated patients had higher insulin secretion at 3 months, but this was not sustained 1 year after surgery. Herein, we report 2 year diabetes outcomes and mechanistic data.
Method: We randomized 43 TPIAT recipients to A1AT (90 mg/kg IV x6 doses, n=13), etanercept (50 mg then 25 mg SQ x 5 doses, n=14), or standard care (n=16). Inflammatory cytokines were drawn at pre-operative screening (x2), pre-islet infusion, and 1,3, 6, 12, 24h, and 3 and 7 days after IAT, including: IP-10, IL-1α, IL-1β, IL-6, IL-8, IL-10, MCP-1, TNF-α. Alpha 1 antitrypsin (A1AT) levels were measured pre-surgery and 7, 14, 21 and 28 days post islet infusion. Islet function was assessed 2 years after TPIAT with mixed meal tolerance test (MMTT), intravenous glucose tolerance test (IVGTT) and glucose potentiated arginine induced insulin secretion (GPAIS).
Results: Participants were adults average 38.5 (SD 12) years old, 17 (40%) males. Cytokine measures, especially IL-6, IL-8, IL-10, and MCP-1, were elevated during and after TPIAT. However, only TNFa differed significantly by treatment, with highest levels in the etanercept group (p=0.027). A1AT levels increased significantly above pre-TPIAT baseline in all 3 groups (p <0.001, Figure 1), with the highest levels in the A1AT-treated group. At 2 years, insulin dependence and measures of insulin secretion by MMTT, IVGTT and GPAIS were similar in all groups (Table1).
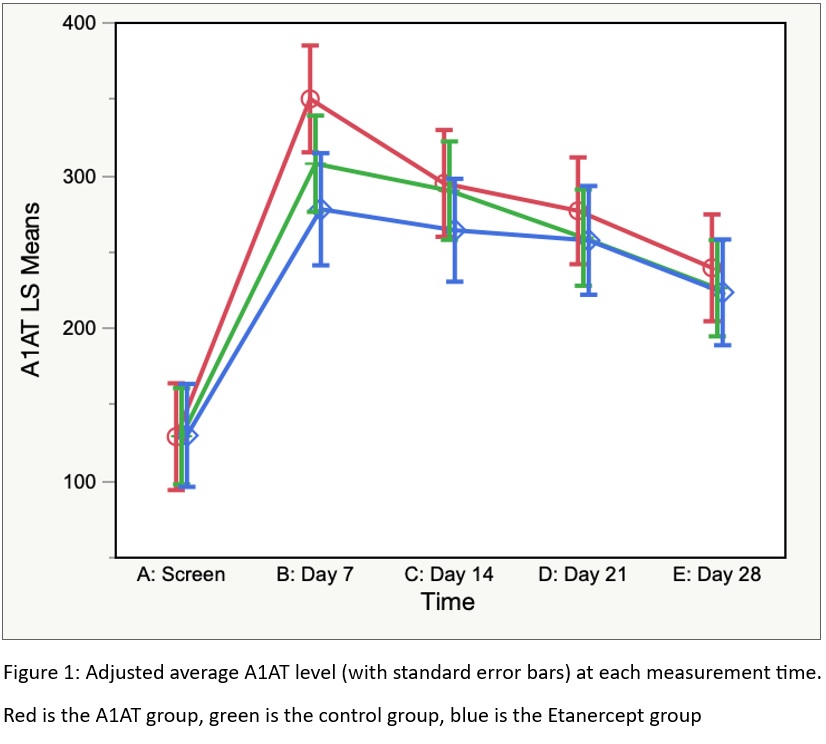
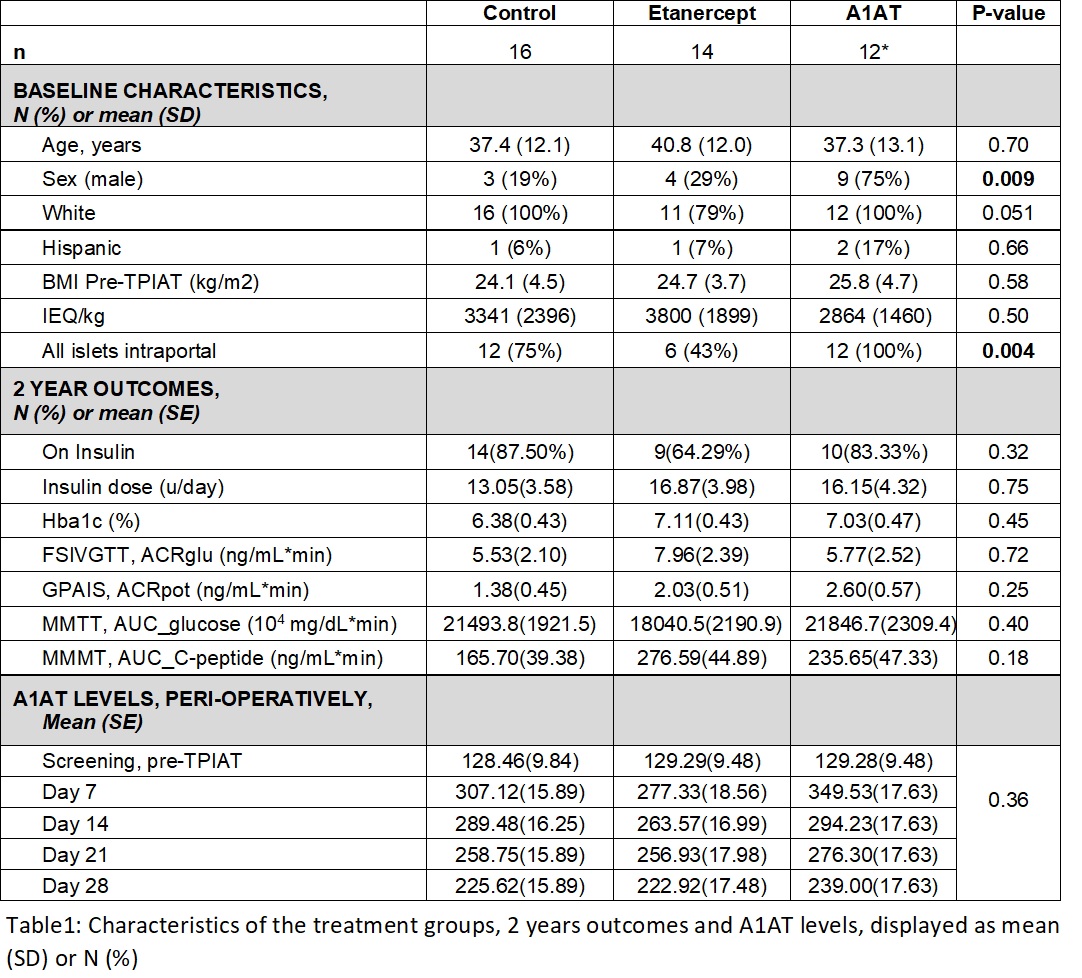
Conclusion: Diabetes outcomes and cytokine profiles (except TNFa) were similar in all groups. Notably, A1AT increased significantly from pre-surgery in all 3 groups, suggesting endogenous upregulation, which may explain the lack of therapeutic benefit from pharmacologic treatment with A1AT.
This study was funded by the National Institute of Health. Grant number R01DK109914 (PI Bellin). .
[1] Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant. Feb 2014;14(2):428-37. doi:10.1111/ajt.12558
[2] Abdel-Karim TR, Hodges JS, Pruett TL, et al. A randomized controlled pilot trial of etanercept and alpha-1 antitrypsin to improve autologous islet engraftment. Pancreatology. Jan 2023;23(1):57-64. doi:10.1016/j.pan.2022.11.006