Pancreatic islets from genetically altered porcine donors are metabolically competent and cure diabetes in mice post xenotransplantation
Lucky Kalekar1, Paola N Perrat1, David M Grosh1, William F Lougee Rodriguez1, Owen J Pearce1, Robert A Policastro1, Leo Waltzer1, Adam Akkad1, Matthew Pandelakis1, Nicholas M Esch1, David Heja1, Kristen Getchell1, Violette Paragas1, Lena Cedrone1, Carey Zamitti2, Michael A Brehm2, Wenning Qin1, Michele E Youd1.
1Development/Immunology, eGenesis, Cambridge, MA, United States; 2Program in Molecular Medicine and the Diabetes Center of Excellence, University of Massachusetts Chan Medical School, Worcester, MA, United States
Introduction: Type 1 diabetes (T1D) is a debilitating autoimmune disease with a heavy healthcare burden that currently does not have a cure. Islet transplantation is a promising therapy for the treatment of T1D, however, there is a lack of sufficient numbers of allogenic human islets to meet the need. Thus, a strong rational exists for the use of porcine donors as a source for islet xenotransplantation. While porcine islets are a potentially unlimited donor source, cross-species immune incompatibility is a major barrier for therapeutic success. Herein, we describe the generation of porcine donors with intentional genomic alterations (IGAs) as a source of metabolically and functionally competent human compatible (HuCoTM) islets.
Methods: HuCoTM islets were derived from porcine donors carrying ten IGAs, including knock-out of three carbohydrate antigens (a-Gal, Sda and Neu5Gc) and insertion of seven human transgenes (CD46, CD55, THBD, PROCR, TNFAIP3, HMOX-1 and CD47). Pancreatic islet-like clusters from one to six-day old neonatal porcine donors were cultured in vitro for seven days. On day 7, neonatal porcine islet (NPI) composition, maturity and transgene expression was assessed using flow cytometry, immunohistochemistry (IHC) and single-cell (sc) RNAseq. Glucose stimulated insulin secretion (GSIS) was used to measure islet function in vitro. The function of the transgenes on islets was evaluated using several transgene specific assays. Finally, a streptozotocin induced diabetes model in NOD SCID gamma (NSG) mice was used to assess the function of NPIs in vivo.
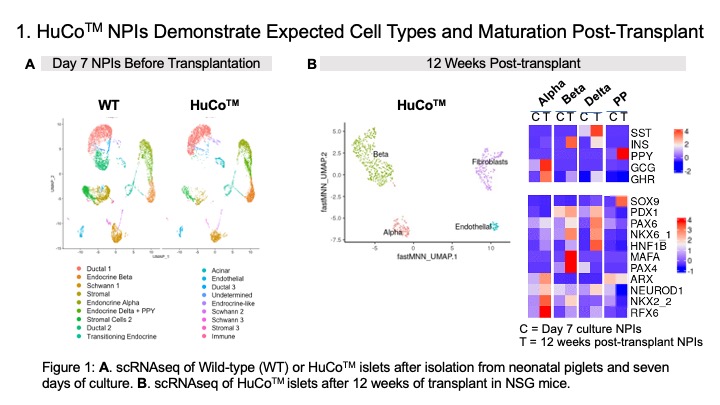
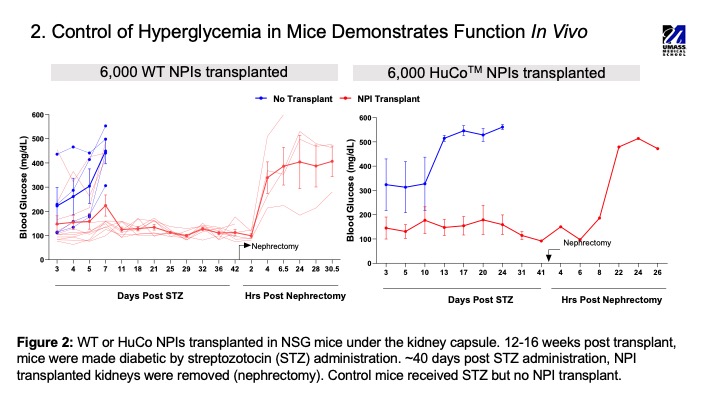
Results: Following the seven-day in vitro culture, HuCoTM NPIs contained 20-30% insulin and 10-15% glucagon expressing cells. NPIs were responsive to glucose challenge in a static GSIS with a stimulation index for insulin >1. All seven human transgenes were expressed by the NPIs. Expression of CD46 and CD55 lead to reduced C3b deposition (>40%) when compared to wild type (WT) islets, while expression of TM and EPCR lead to activated protein C production in a coagulation assay. scRNAseq revealed that most of the cells in the NPIs on day 7 were ductal cell-like, while the beta and alpha cells were genetically immature before transplantation. However, 12-weeks post-transplantation in NSG mice, NPIs had matured, consisting of fully committed endocrine cell populations with insulin producing beta cells being the dominant cell type. Consistent with islet maturation in vivo, transplant of NPIs in diabetic NSG mice lead to a return to normoglycemia by 10-12 weeks after transplantation.
Conclusion: HuCoTM islets express functional transgenes, fully mature in vivo and can correct diabetes in mice.