Replaceable transplant device encapsulating porcine islets improves glycemic control in wild type diabetic mice without immunosuppressant
Kumiko Ajima1, Naoto / Tsuda2, Shigeki / Matsumoto2, Masayuki / Shimoda1.
1Pancreatic Islet Cell Transplantation Project, Research Institute National Center for Global Health and Medicine, Tokyo, Japan; 2Biomaterials Business Division, Mochida Pharmaceutical Co., Ltd., Gotemba, Japan
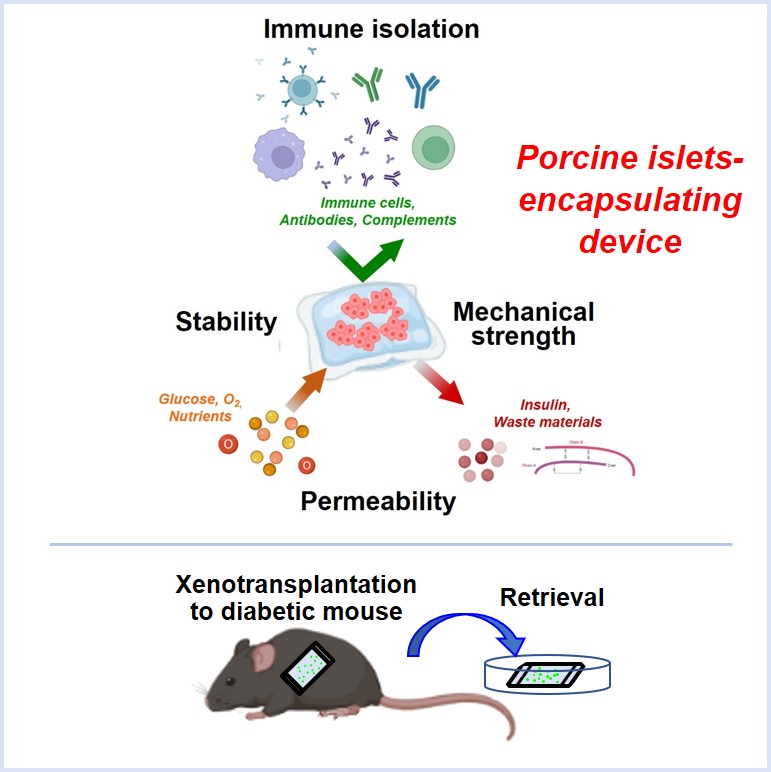
Introduction: Islet transplantation is an effective treatment for type 1 diabetes (T1D). However, donor shortages and the need for immunosuppressants are major challenges. The solution would be to develop a source of insulin-secreting cells and immunoprotective strategies. We have previously developed a novel device with immunoisolation ability for xenotransplantation, and have published that the device encapsulating porcine islets can improve glycemic control for more than 200 days without immunosuppressive drugs when transplanted in immunocompetent diabetic mice (2022 Cell Rep. Methods, K. Ajima et al.) However, the diabetes improvement rate was about 60%, so we improved the fabrication of the device. In this presentation, we report a device that encapsulates porcine islets with a highly stable and permeable hydrogel and a biocompatible immunoisolation membrane.
Method: This device consists of a chemically crosslinked alginate gel and a semipermeable cellulose membrane with immunoisolation ability, and is both flexible and durable. Porcine islets were arranged regularly near the surface inside the sheet hydrogel. Effectiveness of a porcine islet-encapsulating device was evaluated by the changes in non-fasting blood glucose levels in immunocompetent diabetic mice transplanted these devices.
Results: Transplantation of porcine islet-encapsulating devices into the peritoneal cavities of chemically diabetic, immunocompetent mice without immunosuppressants improved glycemic control and the islets remained functional for 3 months. The diabetes improvement rate was increased to 100% compared to existing devices (n=9). Pre-transplantation mean non-fasting blood glucose was 415 mg/dL, which decreased 1 day after transplantation and maintained for 3 months post-transplantation. Three months later, the mean non-fasting blood glucose on the day the device was retrieved was 180 mg/dL. Observation of the device after 3 months showed that it could be easily retrieved with almost no adhesions or fibrosis. Survival porcine islets were observed in the retrieved device, revealing that the devices avoided immune response of xenotransplantation.
Conclusion: The device may provide a safe and functional solution in the development of porcine islet xenotransplantation for T1D.
This work was partially supported by research funds from Mochida Pharmaceutical Co., Ltd..
[1] K. Ajima et al.Cell Reports Methods. 2022; 3: 100370.