Anti-TNFα as an adjunctive therapy in pancreas transplantation
Christophe Masset1, Delphine Kervella1, Benoit Mesnard1, Marine Lorent1, Georges Karam1, Magali Giral1, Claire Garandeau1, Aurélie Houzet1, Clarisse Kerleau1, Simon Ville1, Gilles Blancho1, Jacques Dantal1, Julien Branchereau1, Diego Cantarovich1.
1Center for Research in Transplantation and Translational Immunology, UMR 1064, Institut de Transplantation Urologie Néphrologie , Nantes, France
Introduction. Pancreas graft failure due to thrombosis remains a major complication in the early post-transplant period. We investigated if the adjunction of an anti-TNFα therapy is safe and could improve outcomes within the first year after surgery.
Methods. We retrospectively investigated all DCD pancreas transplantations performed in our institution between 2000 and 2022. Etanercept was prospectively added to our standard immunosuppressive therapy since April 2017 after approval of the local ethic committee. All patients excepted 19 (4%) received rabbit ATG, tacrolimus and MPA or MMF. Steroids were only administered during 7-10 post-operative days. Heparin was systematically given followed by low-dose aspirin. Infections, rejections, and pancreas survival and function were analyzed.
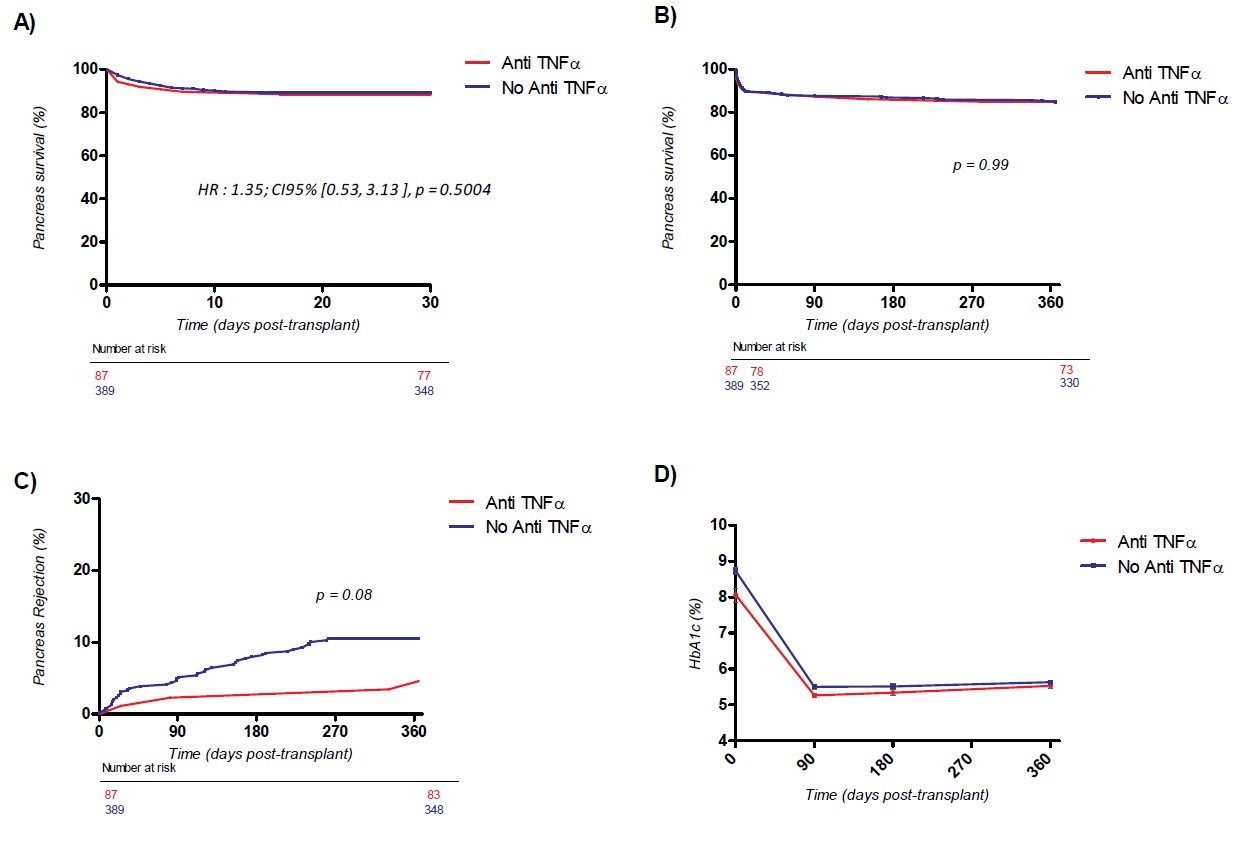
Results. A total of 476 pancreas transplantations (377 SPK, 43 PAK and 56 PTA) were included, among whom 87 received an adjunctive therapy with Etanercept. All alive patients achieved one year follow-up. The use of Etanercept was well tolerated in all patients with no withdrawal. The number and incidence of bacterial, fungal, CMV and BkV infections was identical in both groups. No case of tuberculosis and PTLD was observed. Pancreas survival and function at one and 12 months did not differ between groups in multivariable analysis after adjustment on confounding variables (HR = 1.35; CI95% [0.53;3.13], p = 0.5004). C-peptide at one year was 3.22ng/ml vs 2.97ng/ml; HbA1c was 5.54% vs 5.64%, respectively in the Etanercept and controls. Patients receiving Etanercept presented a lower occurrence of pancreas rejection during the first posttransplant year (4.8% vs 10.5%, p= 0.08). Kidney survival and function was similar in the subgroup of SPK patients.
Conclusion. The use of an induction treatment with anti-TNFα after pancreas transplantation was safe and did not increase in the number of infectious episodes. The number of thrombosis was similar in both groups and pancreas survival was not improved during the first year of transplantation. A reduction in the occurrence of rejections was indeed observed. Combining anti-TNFα and anti-IL1β, as suggested in the field of islet transplantation, will be evaluated in our institution.