Vascularizing a human-scale bioartificial pancreas using sacrificial embedded 3D printing into self-healing alginate
Brenden Moeun1, Maedeh Rahimnejad2, Jonathan A. Brassard3,4, Hamid Ebrahimi Orimi1, Théophraste Lescot6,8, Marc-André Fortin6,8, Steven Paraskevas4, Richard L Leask1,3, Sophie Lerouge2,5, Corinne A Hoesli1,3.
1Department of Chemical Engineering, McGill University, Montreal, QC, Canada; 2Institut de génie biomédical, Université de Montréal, Montreal, QC, Canada; 3Department of Biomedical Engineering, McGill University, Montreal, QC, Canada; 4The Research Institute of the McGill University Health Centre, McGill University, Montreal, QC, Canada; 5Département de génie mécanique, École de technologie supérieure (ÉTS), Montreal, QC, Canada; 6Axe Médecine Régénératrice, Centre de Recherche du Centre Hospitalier Universitaire de Québec (CR-CHUQ), Québec, QC, Canada; 7Centre de Recherche sur les Matériaux Avancés (CERMA), Université Laval, Québec, QC, Canada; 8Département de Génie des Mines, de la Métallurgie et des Matériaux, Université Laval, Québec, QC, Canada
Introduction: With beta cell therapies emerging as a pathway towards a cure for type 1 diabetes, the need to engineer transplantation methods has become increasingly critical. A major limitation in the conception of a bioartificial pancreas is implementing vascularization to achieve sufficient cell survival and function. Layer-by-layer 3D printing offers an avenue to create perfusable artificial tissues, but the need for robust 3D printable materials and lengthy printing times (several hours) limits this approach in its feasibility, scalability, and accessibility. By 3D printing directly into a self-healing, cell-laden bath (embedded 3D printing), inks are provided with mechanical support in situ (Fig. 1), build times are significantly decreased (by ~90%), and more complex, multi-material designs become achievable. Here, we have engineered a self-healing alginate that can be used for embedded 3D printing to rapidly create perfusable bioartificial pancreas tissue.
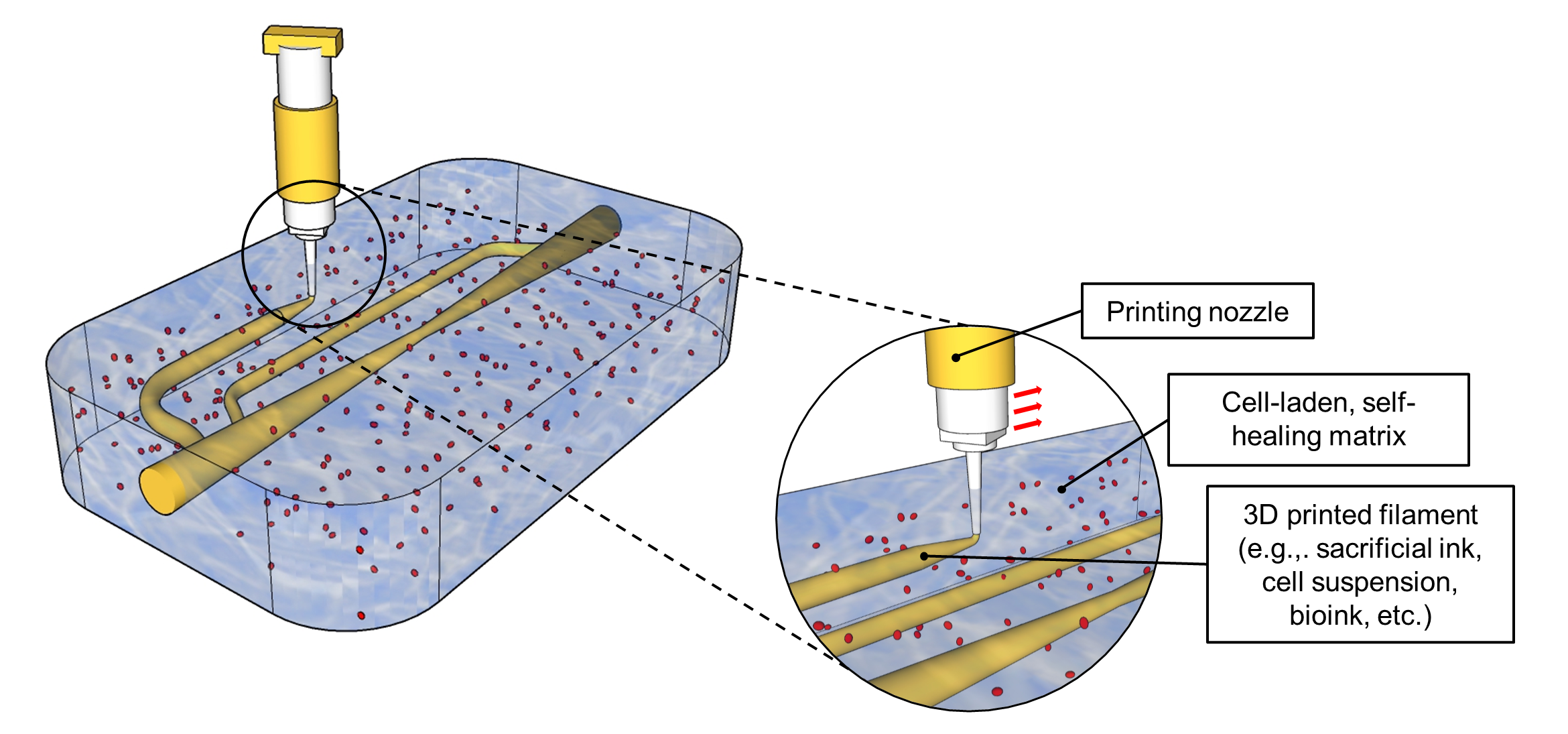
Methods: Self-healing alginates were created by partially gelling with calcium ions and were characterized using oscillatory and rotational rheometry. Pluronic F127 (35% w/v) was used as a sacrificial ink to generate perfusable vascular networks via embedded 3D printing. The viability and insulin secretion response of MIN6 cells were assessed after 2 days of perfusion. The viability of induced pluripotent stem cell (iPSC)-derived pancreatic progenitor aggregates were studied after 5 days of perfusion as they were differentiated from the primitive gut tube to the pancreatic endoderm state.
Results: We show that self-healing matrices can be engineered from alginate by crosslinking it with 10.0 to 25.0 mM of calcium ions. We then measured the self-healing property of these materials by their yield stress (11.0±0.6 to 51.0±3.5 Pa), flow index (0.48±0.01 to 0.53±0.02), and consistency index (12.9±0.5 to 18.3±2.9). Formulations characterized by an Oldroyd number of ~1.52 yielded the most reproducible prints and were conducive to generating multi-branched vascular networks (Fig. 2a). Filament diameters were controlled (0.4-1.8 mm) by varying print speeds. Both MIN6 cells and iPSC-derived pancreatic endoderm cells or aggregates (Fig. 2d) retained high viability immediately after immobilization (≥90%). Following 2 days of perfusion culture, irrigated MIN6 constructs remained viable within a 600±85 μm radius of each channel and were glucose responsive (Fig. 2b and 2c). MIN6 tissues with more complex vascular networks (up to 10 branching channels) remained viable after 2 days of perfusion as well. Similarly, only the iPSC-derived pancreatic progenitor aggregates localized around the perfusion channels remained viable following 5 days of perfusion culture.
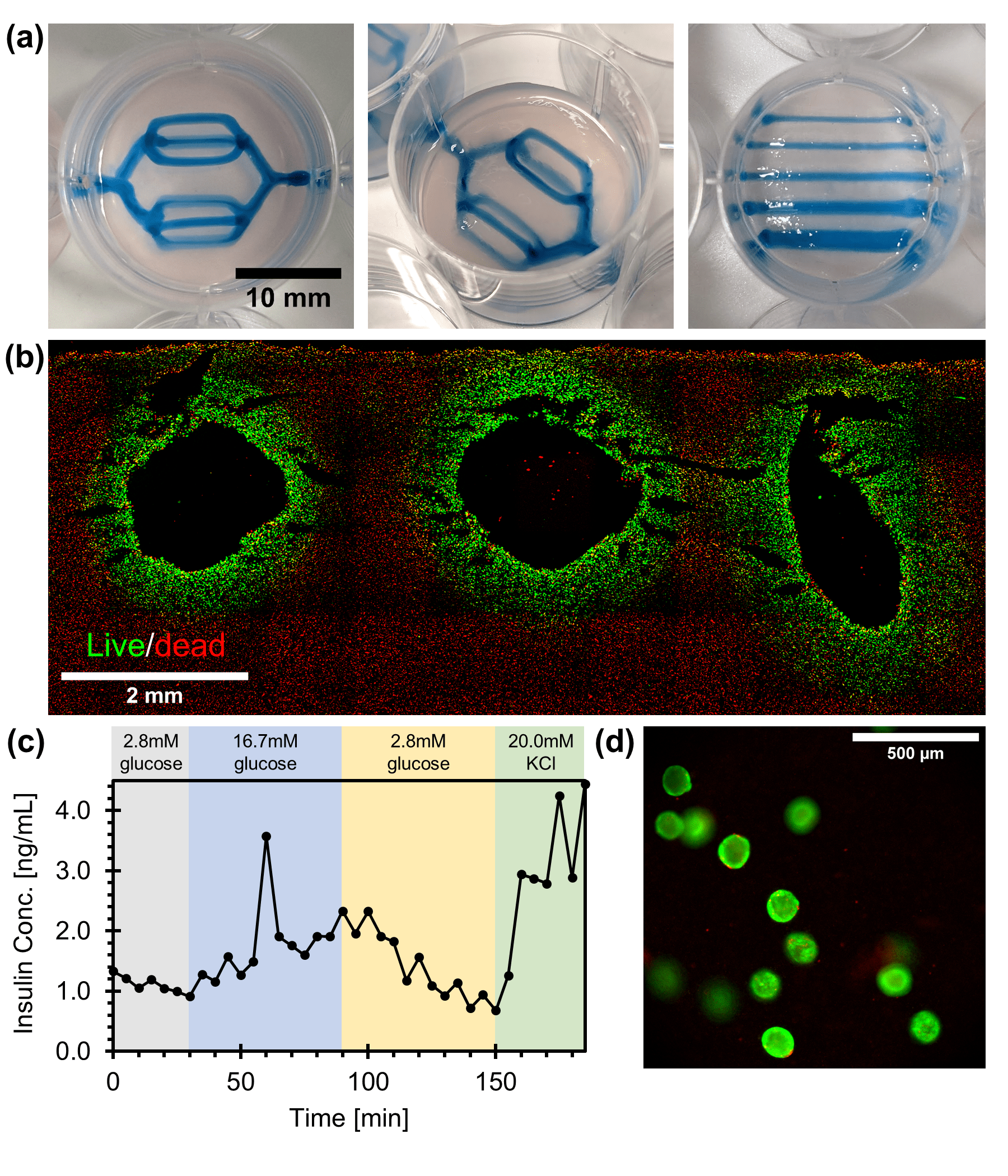
Conclusion: Here we show that we can prepare a self-healing alginate that can be used to streamline the fabrication of perfusable bioartificial pancreas tissues with complex vascular networks.
[1] Moeun, B. N., Da Ling, S., Gasparrini, M., Rutman, A. K., Negi, S., Paraskevas, S., et al. Islet Encapsulation: a Long-Term Treatment for Type 1 Diabetes. Encyclopedia of Tissue Engineering and Regenerative Medicine. 2019; ed. R. L. Reis (Oxford: Academic Press), 217–231.
[2] Skylar-Scott, M. A., Uzel, S. G., Nam, L. L., Ahrens, J. H., Truby, R. L., Damaraju, S., & Lewis, J. A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Science advances. 2019; 5(9), eaaw2459.
[3] Grosskopf, A. K., Truby, R. L., Kim, H., Perazzo, A., Lewis, J. A., & Stone, H. A. Viscoplastic matrix materials for embedded 3D printing. ACS applied materials & interfaces. 2018; 10(27), 23353-23361.