Large-scale long-term 3D-hydrogel culture yields high-quality islets suitable for transplantation
Meirigeng Qi1, Keiko Omori1, Kuan-Tsen Chen1, Jeffrey Rawson1, Nelson Gonzalez1, Lynn Miao1, Shiela Bilbao1, Luis Valiente1, Amber Tucker1, Jacob M Mares1, Kevin Jou1, Janine C Quijano1, Jeffrey S Isenberg1, Teresa H Ku1, Fouad Kandeel1.
1Department of Translational Research & Cellular Therapeutics, Arthur Riggs Diabetes & Metabolism Research Institute of City of Hope, Duarte, CA, United States
Introduction. Islet transplantation (IT) restores insulin production in individuals with severe type 1 diabetes (T1D). However, after isolation, human islets rapidly loose function after isolation and must be transplanted within about two to three days for best outcomes. This limits access to standard IT and combined IT with kidney transplantation. Current methods of cultivating isolated human islets fail to appreciate the role of the extracellular matrix in islet health and do not permit long-term culture. In this study, isolated human islets were cultured in a 3D polysaccharide hydrogel to incorporate an extracellular matrix. Hydrogel stiffness and islet recovery method were optimized and large-scale islet culture study was performed.
Methods. Isolated islets were cultured with 3D-hydrogel in a gas-permeable incubation chamber (100,000 IEQ islets per study at a density of 25,000 IEQ/100 cm2) with CMRL1066 CIT Supplemented Modification islet culture media containing. The culture chamber was incubated under 0.5% CO2 at 37°C. Medium was changed twice weekly. As a comparison, islets from the same donor were maintained under the standard non-hydrogel 2D culture conditions. At fourteen days, the islets were harvested for in vitro and in vivo characterization. Freshly harvested same-donor islets were similarly characterized.
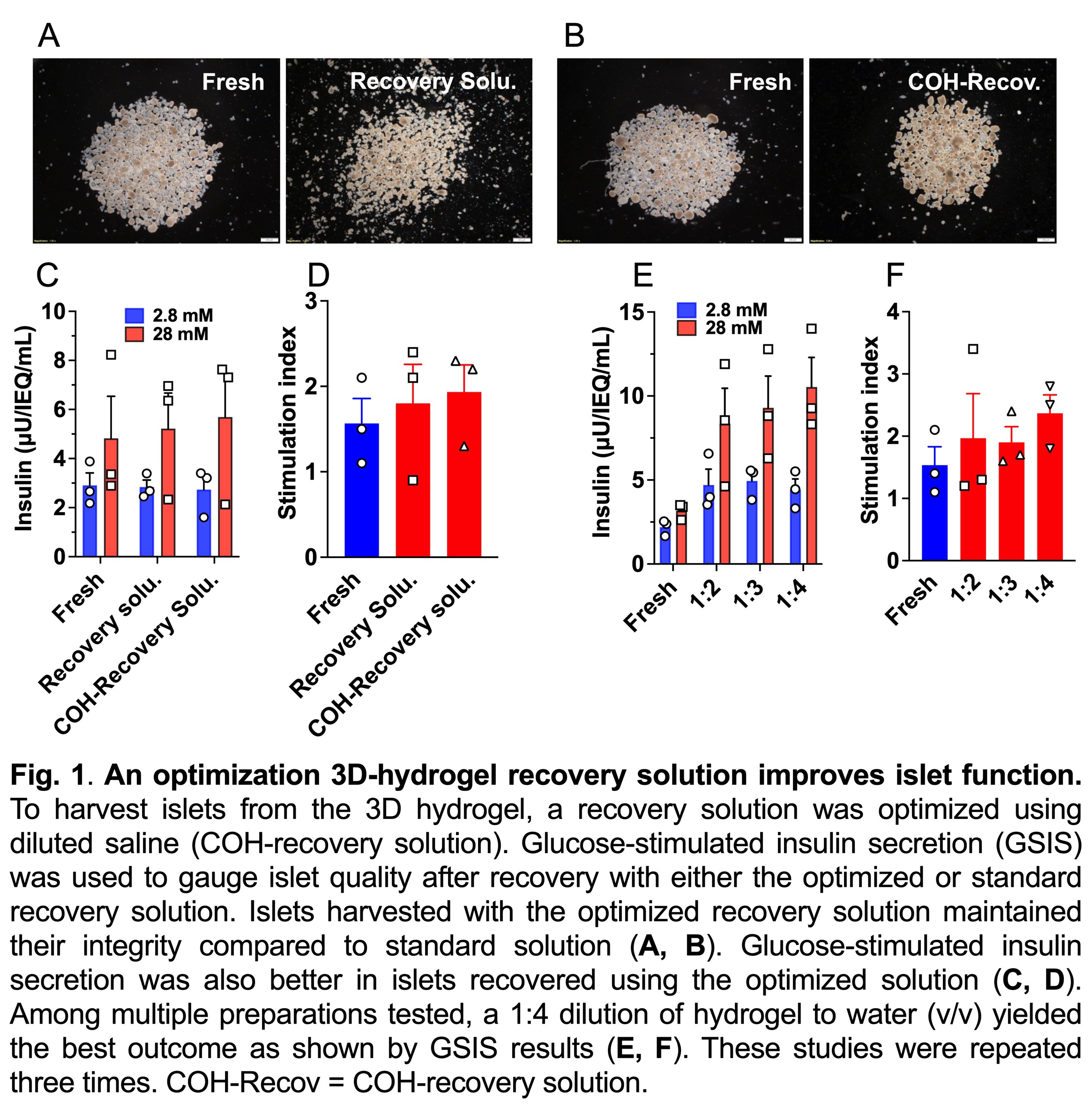
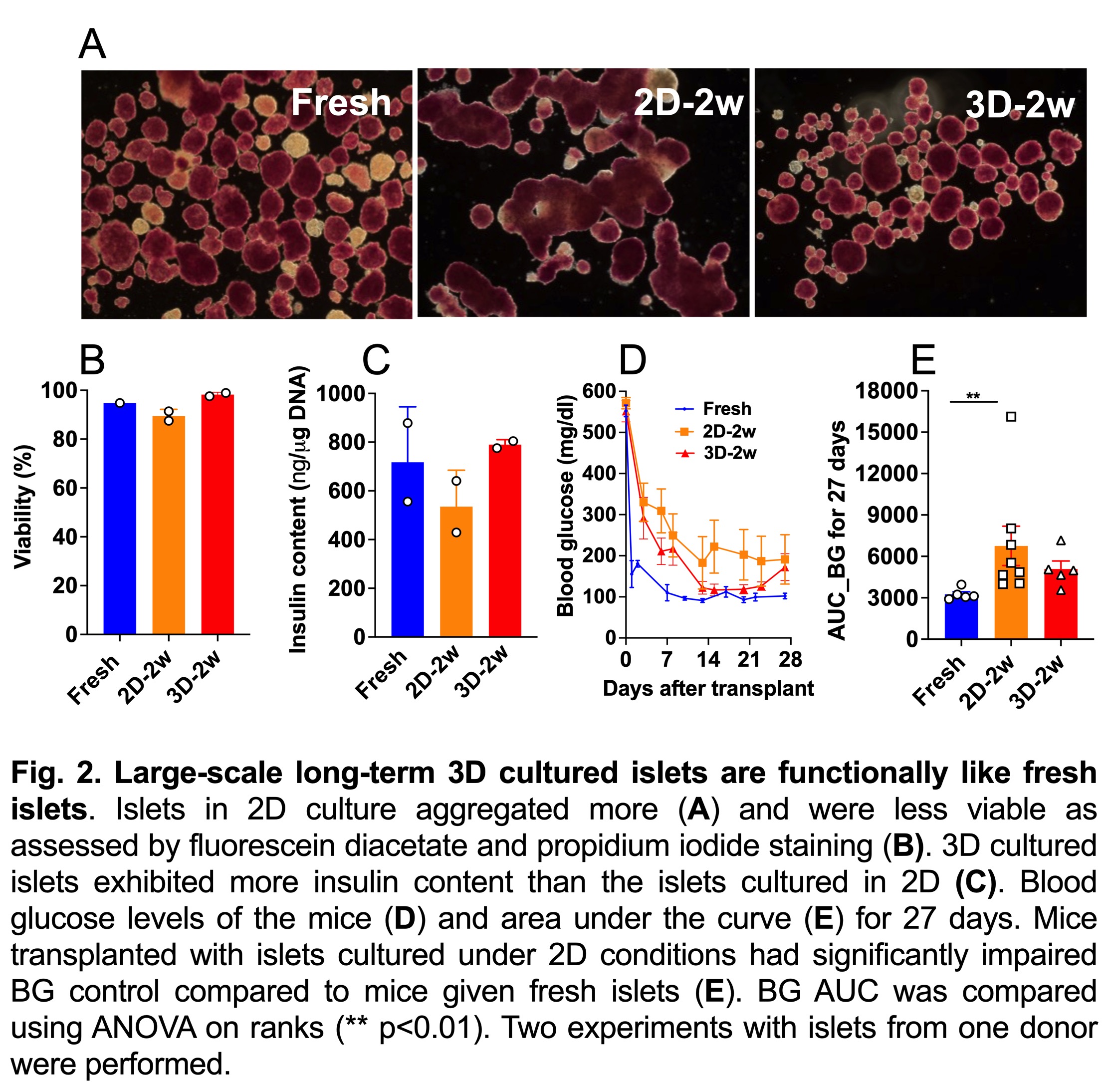
Results. Preliminary data found that COH-recovery solution and 1:4 diluted hydrogel to water solution yielded superior islets as demarcated by glucose-stimulated insulin secretion (Fig. 1). At day 14, 3D-hydrogel cultured islets maintained comparable morphology and viability to fresh islets. In contrast, islets in standard 2D culture were found to aggregate and were less viable (Fig. 2A, B). Notably, insulin content in islets kept in 3D hydrogel was similar to fresh islets and was higher than those kept under 2D conditions (Fig. 2C). Metabolic and functional capacity measured by oxygen consumption rate and dynamic insulin perifusion indicated that large-scale 3D islets were relatively equivalent to fresh islets. In addition, insulin-dependent diabetic mice transplanted with a marginal dose of 3D islets (1200 IEQ/mouse) showed a similar profile of glycemic control to mice given fresh islets, as indicated by the area under the curve analysis of blood glucose levels. In contrasts, mice transplanted with islets cultured under 2D conditions displayed impaired blood glucose control compared to mice given fresh islets (Fig. 2D, E).
Conclusion. Large-scale 3D culture of human islets for 2 weeks yielded high-quality islets that imitate fresh islets in quality and in transplantation study. Our approach has implication in increasing access to IT for patients with T1D.