SEPAX -SEFIA a repurposed closed system for human islet purification and processing in cGMP environment
Naziha Menasria1, Simon Quenneville2, Nathalie Delalleau1,3, Audrey Quenon1, Pauline Petit2, Gianni Pasquetti1,3, Mikael Chetboun1, Thomas Hubert1, Valery Gmyr1,3, Marie Christine Vantyghem1, Francois N Pattou1, Julie Kerr-Conte1,3.
1INSERM U1190, Faculté de Medecine, Université de Lille, Lille, France; 2Cell & Gene Therapy, Cytiva, Eysins, Switzerland; 3Plateforme de Biothrapie, CHU de Lille, Lille, France
Introduction: The COBE 2991 cell processor widely adopted for human islet purification in islet transplant centers, will no longer be available in Europe affecting >17 active transplant centers. Last date for orders is June 2023 with a SUNSET date (end of support for service) in December 2025. Bottle purification of islets exists (open system) and Leiden devised a prototype for islet processing not yet available.
Goal of the study: Demonstrate the proof of concept that the Sepax / Sefia GMP technology single platform, dedicated in stem cell therapy units to hematopoietic stem cell processing in a closed cGMP system, can be repurposed for islet cell processing and purification in a GMP clean room (patent application).
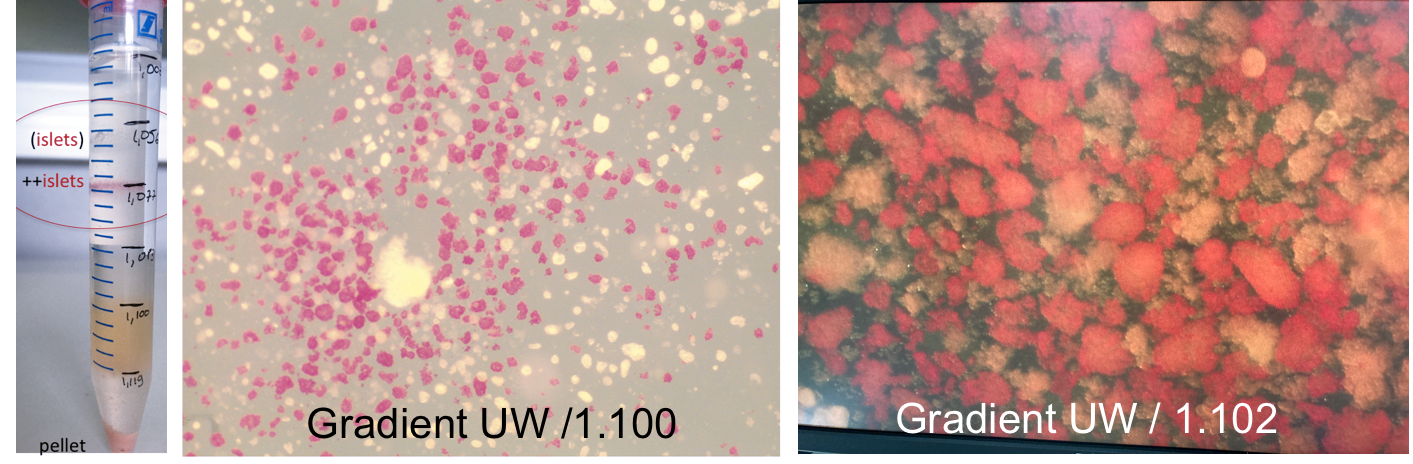
Methods: Islet gradient or Biocoll density gradient purifications were used to purify islets from 7 pig and in 1 human pancreas using the Sepax 2 100 or equivalent Sepax C Pro or Sefia S2000 (Cytiva Europe). Dithizone staining determined islet purity and dithizone+ trypan blue islet viability.
Results: Both discontinuous and continuous density gradients are used to purify islets in the COBE where multiple fractions are recovered and assembled based on purity and pellet volume. In the Sepax, only one standard density gradient is used for mononuclear cell fraction isolation with Neatcell protocol single use processing kit CS-900.2 and one fraction is collected, which appeared not ideal to purify heterogenous human pancreatic tissue. Accordingly, after enzymatic digestion first for each pancreas a test gradient as described in 1994 was performed on 1ml of dithizone pre-stained pancreatic digest to identify the gradients that optimize both recovery and limit transplant volume (<7-8 mls of pellet). Subsequently, the selected density gradients (UW/ 1.077, UW / 1.100, UW / 1.102) were used to purify the pellet in the Sepax yielding 75%(+5% SD) pure fraction of viable islets (> 90%).
Conclusion: We have shown the proof of concept of the purification of human islet cells using Sepax and Sefia closed sytem GMP technology as a strategy to replace COBE 2991 (TRL 1-2-3-4). Secondly, we developed a cooling system to maintani human islets at at <10°C during processing. Application of the technology to clinical grade human pancreases will start in June 2023 in the cell therapy unit (TRL 5-6-7) with an evaluation in vitro and in vivo of product release criteria comparing COBE 2991 purified vs SEPAX/SEFIA purified islets. Validation runs will be submitted for approval before clinical application (TRL 8-9).
Perspectives: Acquisition of cGMP compliant Sepax C Pro together with the Sefia S2000 will enable complete islet processing steps :volume reduction of pancreatic digest, UW pre-soak, 2 simultaneous purifications, washing, and final conditioning of cultured islets in the transplant bag with transplant medium in a closed cGMP system with CE labeling.