Quantitative analysis of microbial contamination in islet isolation for total pancreatectomy and islet auto transplantation (TPIAT): development of bioburden reduction strategies
David Heller1,2, Zachary Swanson1,2, Anna Hance1,2, Rebecca Lawless1,2, Sophie Durant1,2, Siri Larsen1,2, Melena Bellin1,3, Greg Beilman2, Ty Dunn2, Timothy Pruett2, Srinath Chinnakotla2,3,4, Karthik Ramanathan2, Varvara Kirchner2,5, Bernhard Hering1,2, Joshua Wilhelm1,2.
1Schulze Diabetes Institute, University of Minnesota, Minneapolis, MN, United States; 2Department of Surgery, University of Minnesota, Minneapolis, MN, United States; 3Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States; 4Department of Surgery, Hospital of the University of Pennsylvania, Philadelphia, PA, United States; 5Department of Surgery, Stanford Medicine, Stanford, CA, United States
Background: In TPIAT, current final islet product (IP) sterility testing identifies microbial contamination for rapid antibiotic prophylaxis. We sought to (1) quantify “bioburden” (BB) during manufacturing, (2) understand which processes may reduce BB, and (3) develop patient-specific strategies to lessen BB in IP.
Methods: Pancreas preservation solution (PS) and IP sterility were assessed via BACTEC system, growth reported qualitatively (Y/N), and species identified by clinical laboratory. Quantitative data were collected by a Plate Count Method (PCM): PS and IP (n=142), and post-digestion Recombination Solution (RS) and pre-product/COBE (PC) supernatants (n=110) were incubated on TSA plates for 7 days. BB was defined as total CFU from PCM. Donor pancreas fibrosis was stratified as mild/moderate (1-8 out of 10) or severe (9-10).[SL1] To reduce BB, Gentamicin was added to RS (n=91).
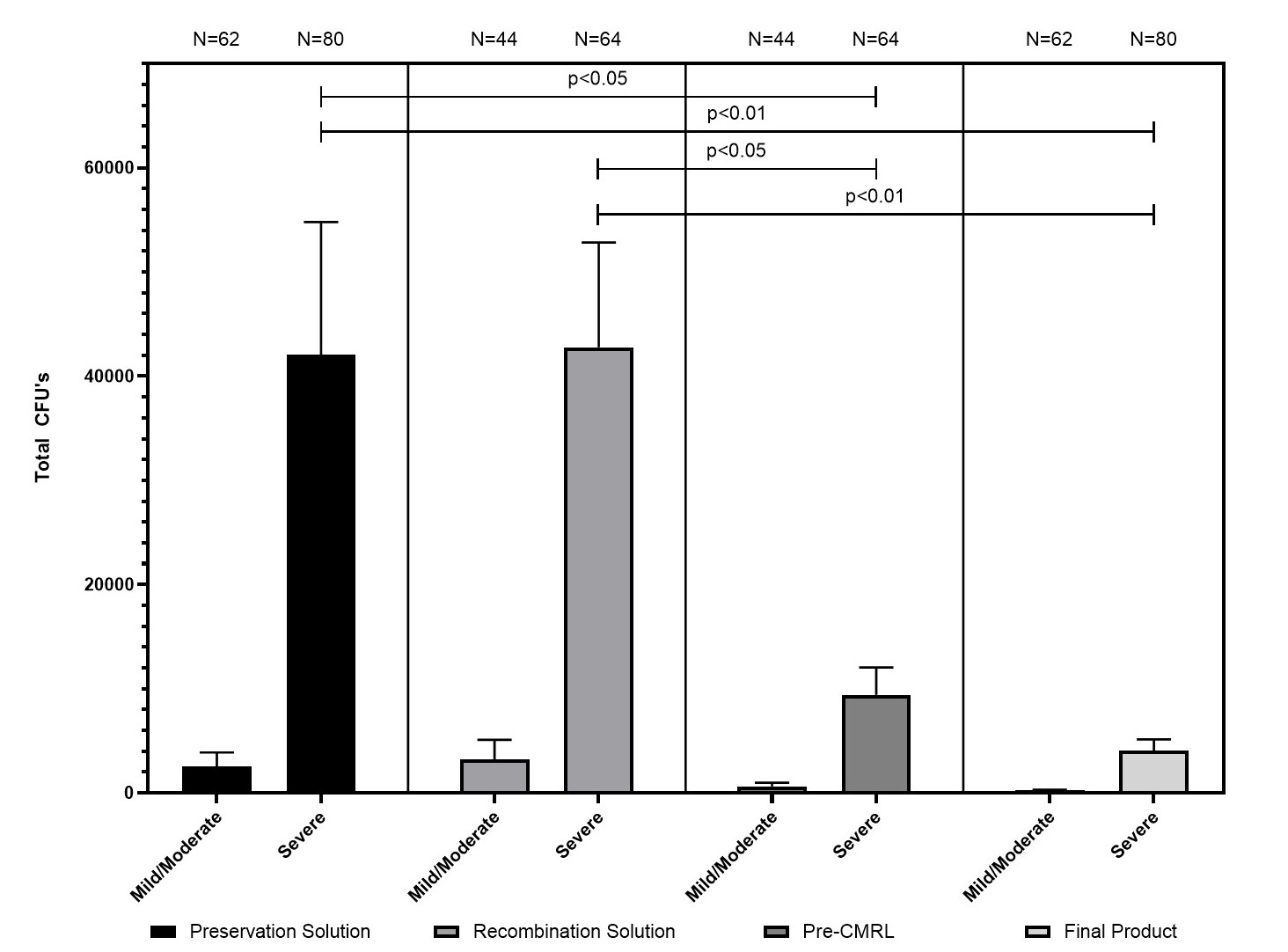
Results: BACTEC and PCM concordance was observed for PS and IP (p<.01), though Granulicatella species were not recoverable by PCM (n=7). In all cases (n=142), mean BB was higher in the severe fibrosis group at all sample time points [PS: 41,552 ± 12,574 vs 2,521 ± 1,364; RS: 42,792 ± 10,065 vs 3,188 ± 1,914; PC: 9,430 ± 2,630 vs 636 ± 361; and IP: 4,070 ± 1,067 vs 229 ± 91, (p<.05) (Figure 1)]. In the severe fibrosis group, mean BB was reduced from PS to PC (p<.05) and IP (p<.01) (Figure 1). Density gradient purification is significantly more likely in both mild and moderate cases vs severe (p<0.001) due to high tissue volume. BB is significantly lower in cases requiring purification for all timepoints (p<0.001). When excluding purified cases, fibrosis is still a predictive factor of increased BB in PS, RS, PC, and IP (p<0.01). In a pilot study, addition of gentamicin to the RS resulted in significant reduction IP sterility positive (n= 41 p<0.05). No significance was observed after 50 subsequent cases. Initial subset analysis (fibrosis, BB, positive PS, purification) were not significant.
Conclusion: Although BB varies remarkably between patients, we characterized a subset with the highest IP BB (fibrosis ≥9, unpurified) where BB reduction may be inadequate. Cases requiring purification have lower BB, but not due to the purification process. BB reduction is inherit to islet manufacturing due to high media volumes and extensive washing steps. Current dilution during washing steps is ~1x106-fold from PS to IP; further dilution offers potential to reduce BB in high risk patients. A proposed novel method to reduce BB involves additional washes to dilute IP an added 1x104 and requires <20 minutes of processing. Further study is needed to identify high risk subsets and evaluate BB reduction strategies.
[1] Berger MG, et al., Microbial contamination of transplant solutions during pancreatic islet autotransplants is not associated with clinical infection in a pediatric population, Pancreatology (2016), http://dx.doi.org/10.1016/j.pan.2016.03.019.