Synergistic inhibition of human NK cell activation by co-expression of HLA-E and HLA-G on porcine endothelial cells
Arthur A Cross-Najafi1, Kristine Farag1, Abdulkadir Isidan1, Wei Li2, Wenjun Zhang1, Zhansong Lin3, Julia R Walsh1, Kevin J Lopez1, Yujin Park1, Nancy G Higgins4, David K.C. Cooper Cooper5, Burcin Ekser1, Ping Li1.
1Surgery, Indiana University School of Medicine, Indianapolis, IN, United States; 2Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN, United States; 3Ragon Institute of MGH, MIT, and Harvard, Charlestown, MA, United States; 4Indiana University Health, Indianapolis, IN, United States; 5Center for Transplantation Sciences, Massachusetts General Hospital/Harvard Medical School, Charlestown, MA, United States
Introduction: Natural killer (NK) cells play an important role in immune rejection in solid organ transplantation. Introducing inhibitory ligands on xenografts via genetic engineering may mitigate human xenoreactive NK cell activation and improve xenograft survival. NK cell inhibitory ligands such as non-classical human leukocyte antigens (HLA)-E and -G are highly expressed in the human placenta and contribute to establishing and maintaining immune tolerance at the maternal-fetal interface. Introducing HLA-E or HLA-G on porcine cells to attenuate human NK cell activation via the distinct inhibition pathways have been reported. This study investigates the role of co-expression of HLA-E and HLA-G on porcine endothelial cells in inhibiting human NK cell activation.
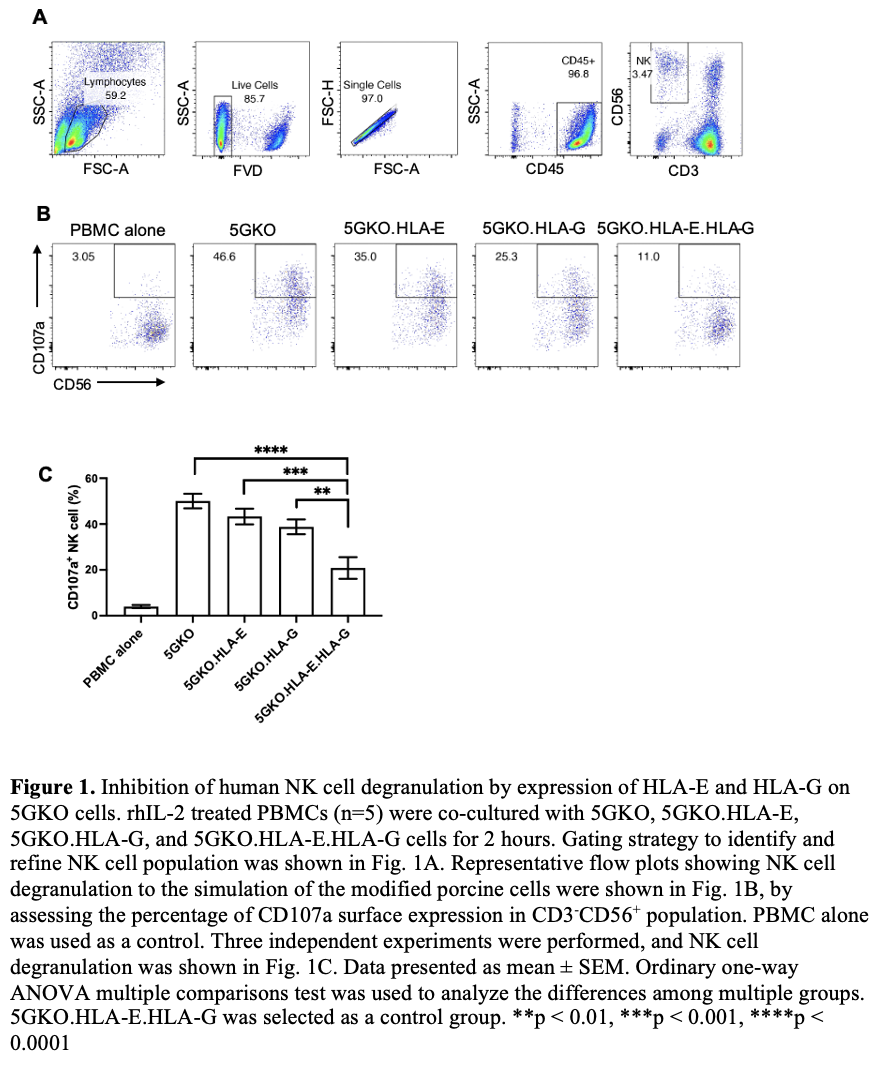
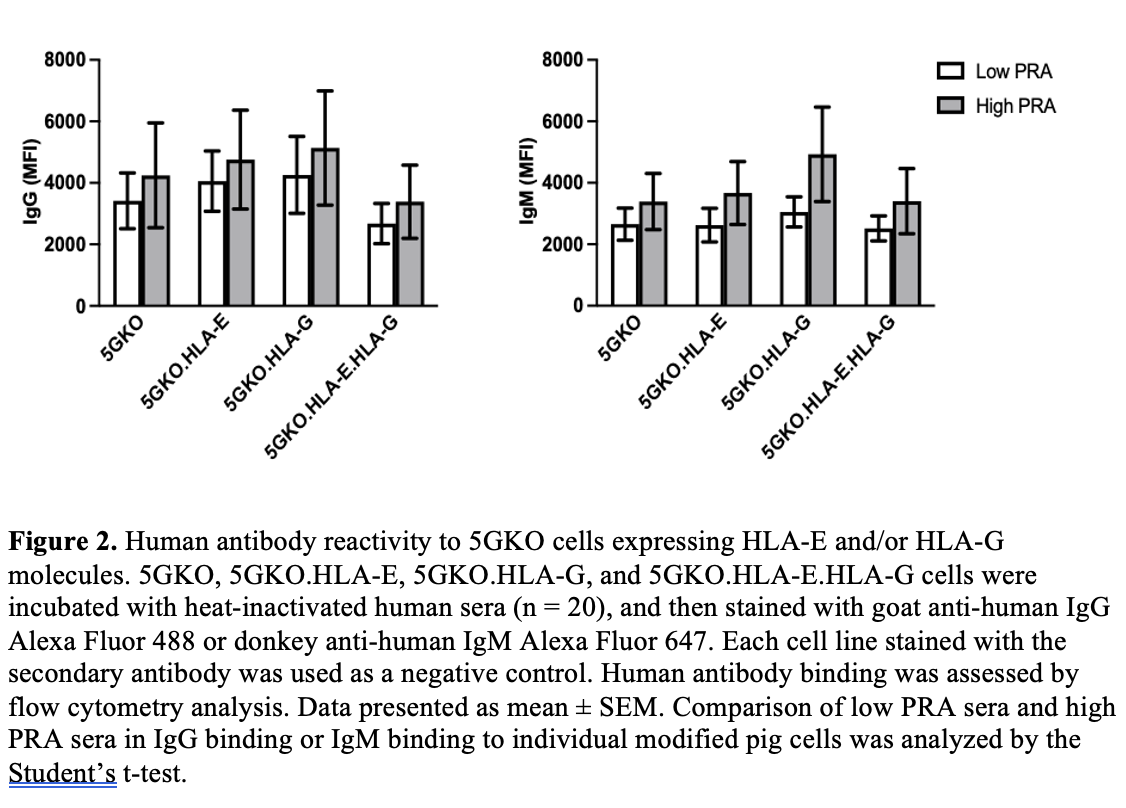
Methods: HLA-E or HLA-G gene along with beta-2-microglobulin gene were engineered and introduced in a 5-gene knockout porcine endothelial cell line (5GKO, GGTA1/CMAH/β4galNT2/SLA-I α chain/β-2 microglobulin) by electroporation. Transfected porcine cells were cultured in selection media. HLA-E and/or HLA-G positive cells were isolated by fluorescence-activated cell sorting. Human NK cell degranulation was assessed by flow cytometry analysis of CD107a positive cells in CD3-CD56+ population from human blood peripheral mononuclear cells. CD107a is a sensitive marker of NK cell activation and correlates with NK cell cytotoxicity. Human sera were obtained from patients on the kidney transplant waitlist, 10 sera with high panel reactive antibody (PRA) and 10 sera with low PRA, for a total of 20 samples (n = 20). Human IgG and IgM antibody reactivity to HLA-E and/or HLA-G on pig cells was examined by flow cytometry analysis.
Results: We found that HLA-E expression was much higher on pig cells co-expressing HLA-E and HLA-G compared to pig cells expressing HLA-E alone. Pulsing exogenous HLA-G leader peptide (VMAPRTLFL) could enhance HLA-E expression on pig cells. Co-expression of HLA-E and HLA-G on porcine cells led to a significant reduction in human NK cell degranulation compared to the cells expressing HLA-E or HLA-G alone and the parental cell line. HLA-E and/or HLA-G on pig cells did not show reactivity to human serum IgG and IgM antibodies.
Conclusions: This in vitro study demonstrated for the first time that co-expression of HLA-E and HLA-G on porcine endothelial cells provided superior inhibition in human xenoreactive NK cells, which may guide future genetic engineering of pig with improved human-pig immune compatibility.
NIH NIAID R21AI164002 (PL) . Ralph W. and Grace M. Showalter Research Trust (PL). The Board of Directors of the Indiana University Health Values Fund for Research Award (VFR-457-Ekser). The Indiana Clinical and Translational Sciences Institute, funded in part by Grant # UL1TR001108 from the National Institutes of Health (NIH).