Hypoxia-related genes in pre-transplant human islets as potential markers for the transplantation outcomes in diabetic mice
Hiroyuki Kato1, Mayra Salgado1, Daniel Mendez1, Nelson Gonzalez1, Jeffrey Rawson1, Janine Quijano1, Doreen Ligot1, Bennie Balandran1, Chris Orr1, Keiko Omori1, Meirigeng Qi1, Ismail Al-Abdullah1, Yoko Mullen1, Hsun Teresa Ku1, Fouad Kandeel1, Hirotake Komatsu1.
1Department of Translational Research & Cellular Therapeutics, Arthur Riggs Diabetes & Metabolism Research Institute of City of Hope, Duarte, CA, United States
Background: Pre-transplant quality assessments of the isolated islets are critical to predicting transplantation outcomes for type 1 diabetes. Although islet viability, morphological scoring, and oxygen consumption rate are reported as potential markers for successful human islet transplantations into diabetic immunodeficient mice [1, 2], identifying additional biomarkers is needed for accurate prediction. Since islets are susceptible to hypoxia, we hypothesized that the expressions of hypoxia-related genes in pre-transplant isolated islets, reflecting the intra-islet oxygen microenvironment, could be the potential predictive markers for the transplantation outcomes.
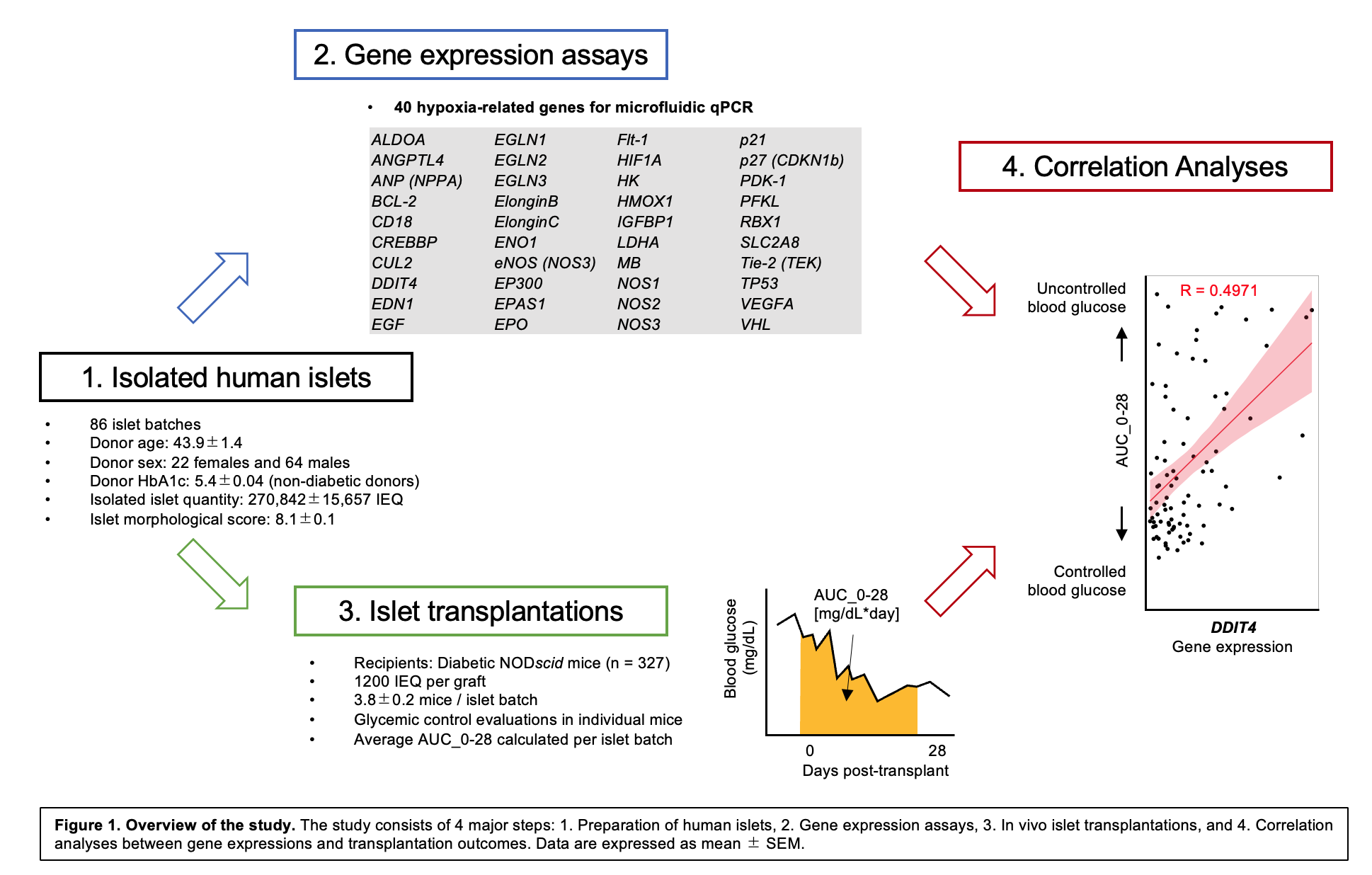
Methods: An overview of the study is shown in Figure 1. Human islets from deceased donors were isolated at City of Hope (n = 86 batches). Islets were transplanted under the kidney capsule of streptozotocin-induced diabetic NOD-scid mice (1200 IEQ per graft, 327 mice). Post-transplant glycemic control was assessed quantitatively using the area under the curve (AUC) of blood glucose from days 0–28 (AUC_0-28 [mg/dL*day]) in individual mice [1] and averaged per islet batch. Note that low AUC_0-28 indicates controlled post-transplant blood glucose level, and low AUC_0-28 is highly correlated to the diabetes reversal in mice [1]. Microfluidic qPCR of 40 hypoxia-related genes was employed using RNA from pre-transplant islets, and gene expressions were normalized to a housekeeping gene. Correlations between gene expressions and AUC_0-28 were calculated using linear regression. Multiple regression analysis was further employed to generate an equation for predicting the transplantation outcomes.
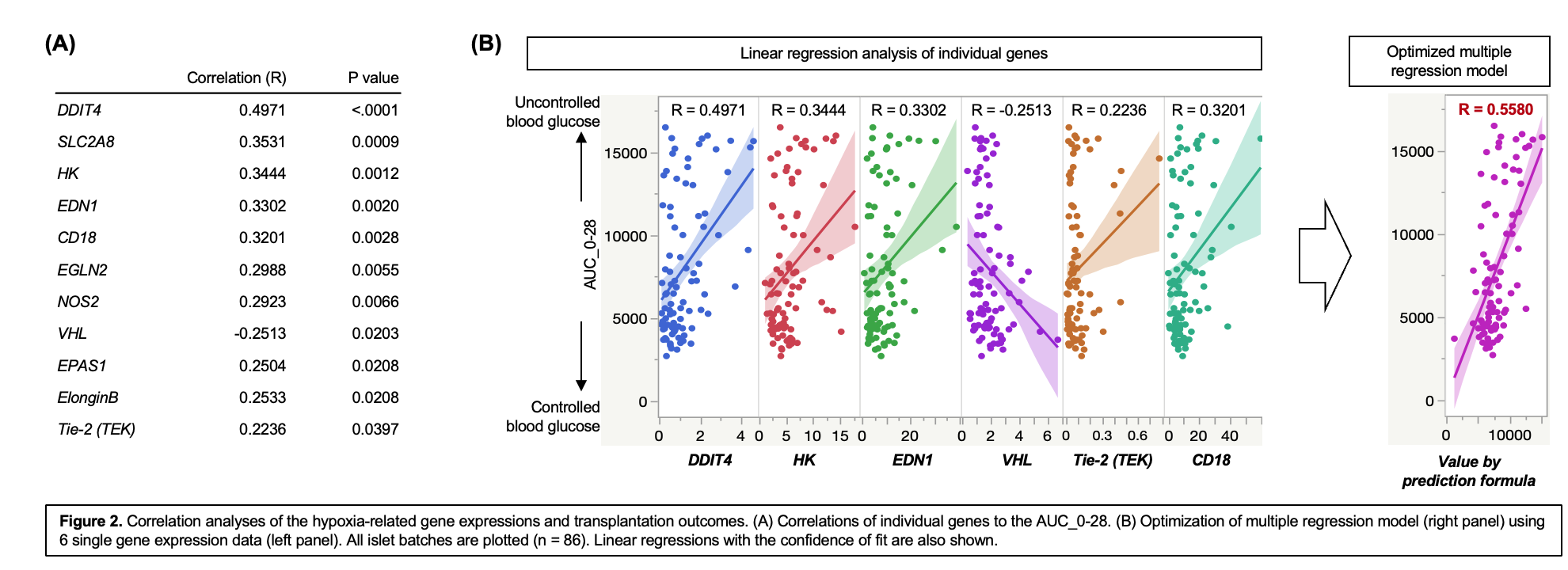
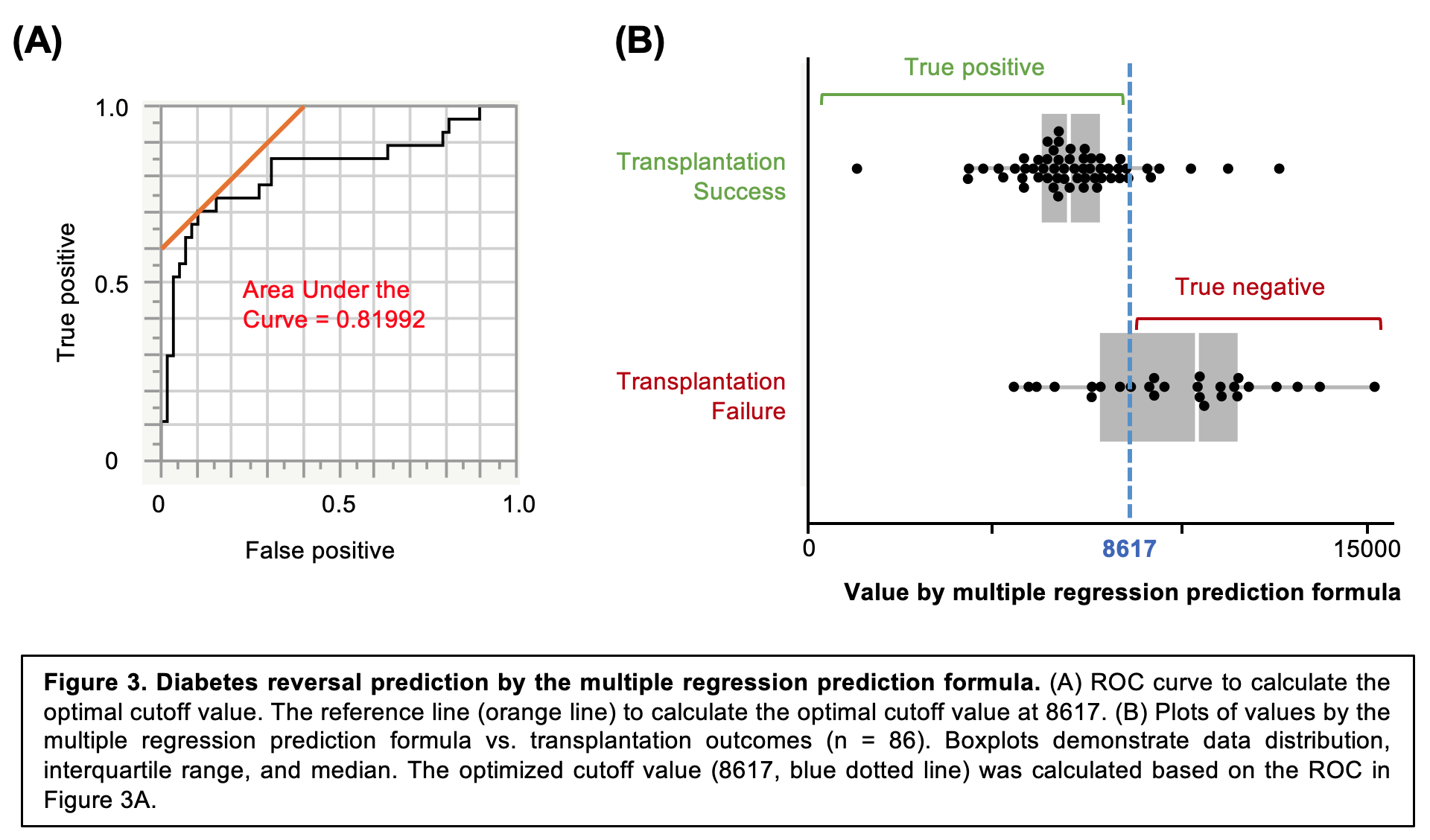
Results: Among 40 genes examined, 11 genes were significantly correlated to the transplantation outcome, (Figure 2A). Of note, DDIT4 expression demonstrated the highest correlation to the transplantation outcomes (R = 0.4971, P <0.0001). Further, we generated the prediction formula using multiple regression model with 6 genes (Prediction value = 7111 + 62*CD18 -116*EDN1 + 313*HK + 5059*Tie-2 - 1295*VHL + 1177*DDIT4), which demonstrated the improved correlation to transplantation outcome (R = 0.5580, Figure 2B). Lastly, we applied the prediction formula to predict diabetes reversal (qualitative factor), which calculated a high AUC of Receiver Operating Characteristic (0.81992, Figure 3A), with high sensitivity at 70.4% and specificity at 90.0% in predicting transplantation success (Figure 3B).Conclusions: Hypoxia-related genes of pre-transplant human islets are promising prediction markers for islet transplantation outcomes in diabetic mice. Notably, a multiple regression model of hypoxia-related genes was highly predictive of transplantation outcomes.
We thank the manufacturing team at the Southern California Islet Cell Resources Center, Arthur Riggs Diabetes & Metabolism Research Institute of City of Hope, for preparing the isolated human islets. This work was supported by Nora Eccles Treadwell Foundation to HKo. We thank Sung Hee Kil, Ph.D. for critical reading and editing of the manuscript. .
[1] Salgado M, Gonzalez N, Medrano L, Rawson J, Omori K, Qi M, et al. Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model. Cell transplantation. 2020; 29: 963689720919444.
[2] Komatsu H, Qi M, Gonzalez N, Salgado M, Medrano L, Rawson J, et al. A Multiparametric Assessment of Human Islets Predicts Transplant Outcomes in Diabetic Mice. Cell transplantation. 2021; 30: 9636897211052291.