Exploring local immune modulation with rapamycin-eluting microparticles to preserve islet graft function in mice
Jordan Wong1,2, Purushothaman Kuppan1,2, Jessica Worton1,2, Joy Paramor1,2, Chelsea Castro1,2, Karen Seeberger1,2, Kateryna Polishevska1,2, Gregory S Korbutt1,2, Andrew R Pepper1,2,3.
1Department of Surgery, University of Alberta, Edmonton, AB, Canada; 2Alberta Diabetes Institute, University of Alberta, Edmonton, AB, Canada; 3Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada
Background: Islet transplantation is an effective means for a subset of people living with type 1 diabetes to achieve insulin independence; however, lifelong systemic immunosuppression required to subvert the immune response remains a major barrier to patient inclusion. Herein, we explore the use of a localized drug delivery system to preserve murine islet allograft function, reducing the need for toxic systemic immunosuppression.
Our previous work demonstrated that localized delivery of dexamethasone eluting microparticles reduced intragraft proinflammatory cytokine expression and prolonged the function of murine islet allografts. Thus, we present our efforts in optimizing a microparticle formulation that encapsulates rapamycin (rapa), a more potent immunosuppressant employed in clinical islet transplantation. Localized delivery of rapa may be an attractive strategy to provide islet allograft protection while minimizing the diabetogenic effects of high-dose systemic rapa.
Methods: Rapa was encapsulated via a modified single emulsion solvent evaporation technique. Utilizing Food and Drug Administration-approved poly(lactide-co-glycolide) (PLGA), we formed microparticles with an 87.3 ± 2.1 % rapa encapsulation efficiency. We assessed the in vitro and in vivo effects of our rapa-eluting microparticles on islets with the Seahorse XF24 assay and murine islet transplant models, respectively. Rodents with streptozotocin-induced diabetes were transplanted with microparticles and islets isolated from naïve BALB/c mice. Recipients included BALB/c (syngeneic) and C57BL/6 (allogeneic) mice.
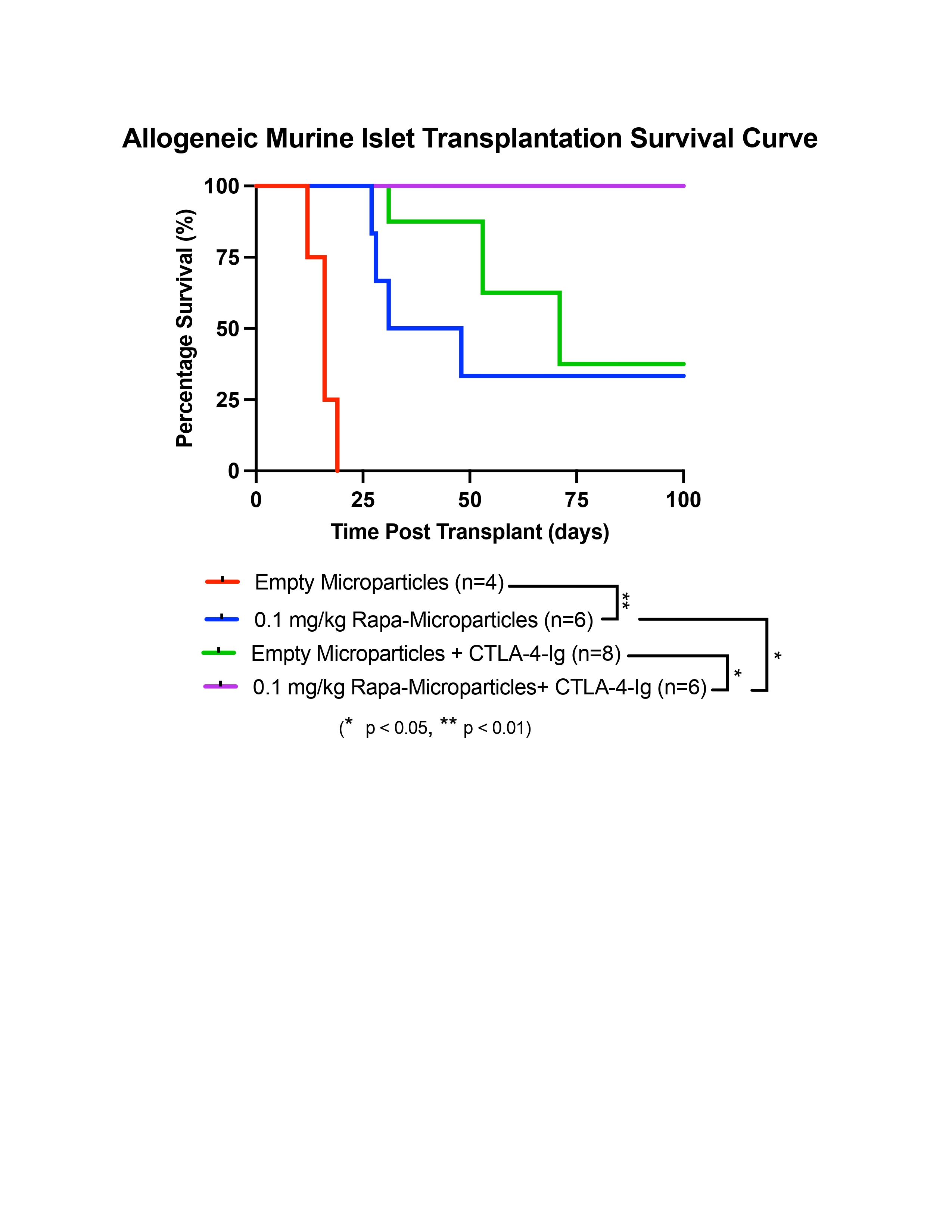
Results: Microparticles showed sustained in vitro rapa release for up to 35 days. Human islets co-cultured 24 hrs with rapa-microparticles (1.0 and 2.0 mg) demonstrated comparable mitochondrial potency and glucose-stimulated respiration to untreated islets, while 25 nM rapa incubation blunted the glucose response and increased proton leak. Syngeneic islets co-transplanted with a 0.1 mg/kg dose of rapa-microparticles (1.0 mg; n=3) under the kidney capsule all achieved euglycemia and a 0.2 mg/kg rapa-microparticles (2.0 mg; n=8) demonstrated partial graft function (3 of 8). Islet allograft recipients receiving 0.1 mg/kg rapa-microparticles (n=6) demonstrated prolonged allograft survival compared to empty microparticles recipients (n=4; P<0.01). Rapa-microparticles + CTLA-4-Ig (n=6) resulted in 100% allograft survival at 100 days compared to 38% empty microparticles + CTLA-4-Ig (n=8; P<0.05).
Conclusion: Our novel rapa-eluting microparticles prolonged allograft function and worked synergistically with CTLA-4-Ig therapy. Further validating our findings in a humanized mouse model can help us study rapa-microparticles tolerogenic effects in the context of the human immune system. Collectively, localized drug delivery has the potential to alter the immune environment, protect grafts, and may serve as a safe adjuvant approach in clinical islet transplantation.
Juvenile Diabetes Research Foundation (2-SRA-2019-779-S-B) . Juvenile Diabetes Research Foundation (JDRF) Career Development Award (5-CDA-2020-945-A-N) . ARP is also a Canada Research Chair in Cell Therapies for Diabetes, and as such, this research was undertaken, in part, thanks to funding from the Canada Research Chairs Program.