Jagan Kalivarathan, United States has been granted the TTS-IPITA Congress Scientific Award
Surface modification of Bissulfosuccinimidyl suberate as an encapsulation strategy for islet transplants
Jagan Kalivarathan2, Prathab Balaji Saravanan1, Shujauddin Mohammed1, Brian Fuglestad3, Soma Dhakal3, Marlon F Levy2, Mazhar A Kanak1.
1Department of Surgery, Virginia Commonwealth University, Richmond, VA, United States; 2Hume Lee Transplant Center, VCU Health System, Richmond, VA, United States; 3Department of Chemistry, Virginia Commonwealth University, Richmond, VA, United States
Purpose: Early post-transplant inflammation has been a significant factor resulting in the loss of more than 50% of pancreatic islets, affecting long-term islet transplant outcomes. Control of early inflammatory events could improve the efficacy of islet cell transplantation and may enhance the long-term survival of islet grafts. Cell surface modification is an attractive method for masking cell features that may instigate inflammatory cascades. Bissulfosuccinimidyl suberate (BS3) is a homobifunctional crosslinker with NHS ester on each side to bind to amine groups. Here we have evaluated the surface conjugation potential of BS3 which may be used to immobilize anti-inflammatory compounds onto the surface of the islets for local delivery to the graft microenvironment.
Methods: Human islets (2000IEQ) were surface conjugated with BS3 in different concentrations (0.5 mM, 1 mM, and 5 mM). Visual coverage and efficiency of surface conjugation were determined by incorporating an Amine-PEG-Biotin conjugate. Surface-conjugated islets were then cultured for 7 days. Visualization of conjugation and viability was performed on days 0, 1, 4, and 7 of culture by staining islets with Streptavidin FITC, Hoechst33314, and propidium iodide. The impact of BS3 conjugation on islet function was determined by measuring the oxygen consumption rates (OCR) and glucose-stimulated insulin secretion (GSIS).
Results: The modification of islets with BS3 was found to be exceptionally biocompatible. The viability and functional integrity of islets was not altered at 0.5 mM and 1 mM BS3, but viability showed a mild decline at 5 mM BS3. However, improved conjugation of the islet surface and good coverage were noted in the 5 mM BS3 group. The surface modification was maintained for all 7 days tested with minimal loss of coverage. Figure 1b shows the surface coverage of islets with BS3 on day 7.
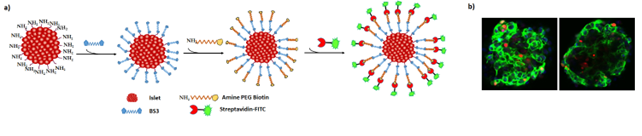
Conclusion: Surface modification of islets with BS3 is a safe and tolerable approach that may be used for localized drug delivery strategies. This approach opens new opportunities for the delivery of bioactive molecules such as anti-inflammatory and immunomodulatory nano-drugs locally in the islet transplant microenvironment.
This work was supported by U.S. Department of Defense (Grant#PR211164).