
Clinical trial protocol for porcine islet xenotransplantation in South Korea
Byung Joon Kim1, Jun-Seop Shin2, Byoung-Hoon Min2, Jong-Min Kim3, Chung-Gyu Park3,4, Hee-Jung Kang5, Eung Soo Hwang4, Won-Woo Lee4, Jung-Sik Kim4, Hyun Je Kim4, Iov Kwon6, Jae Sung Kim7, Geun Soo Kim7, Joonho Moon7, Du Yeon Shin7, Bumrae Cho7, Heung-Mo Yang7, Sung Joo Kim7, Kwang-Won Kim1.
11Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University , Incheon, Korea; 2Tascom, Co. Ltd, Tascom, Co. Ltd, Gyeonggi-do , Korea; 3Transplantation Research Institute, Seoul National University College of Medicine, Seoul, Korea; 4Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea; 5Department of Laboratory Medicine, Hallym University College of Medicine, Anyang, Korea; 6Department of Medical Education, Ewha Womans University College of Medicine, Seoul, Korea; 7GenNBio, Inc, GenNBio, Inc, Kyeonggi-do, Korea
Islet transplantation is a promising treatment option for patients with type 1 diabetes who suffer from labile glycemic control and frequent hypoglycemic unawareness. However, the limited availability of human donors has hindered its widespread use. Porcine islets have long been considered a viable replacement for human pancreatic islets. Although clinical trials of encapsulated porcine islets have shown long-term clinical improvements, the use of naked porcine islets combined with immunosuppression has reported limited short-term clinical benefits. This discrepancy may be due to the lack of prior preclinical experiments showing efficacy in pig-to-nonhuman primate (NHP) transplantation settings. To address this, the International Xenotransplantation Association has mandated the need for efficacy and safety data from NHP studies, as outlined in 2009 and updated in 2016 consensus statements. Our research group has successfully met the rigorous preclinical criteria in NHPs, making us the only group to do so. Based on our preclinical results, we submitted an investigational new drug (IND) application for the first human clinical trial to the Korean Ministry of Food and Drug Safety (MFDS) in 2020. After extensive discussion and revisions, the clinical trial protocol was approved in December 2022. In this poster, we present an overview of the clinical protocols and the process we went through to get the proposal approved. We hope that our experiences will be helpful for other scientists seeking to expedite similar human clinical trials of porcine islet xenotransplantation worldwide.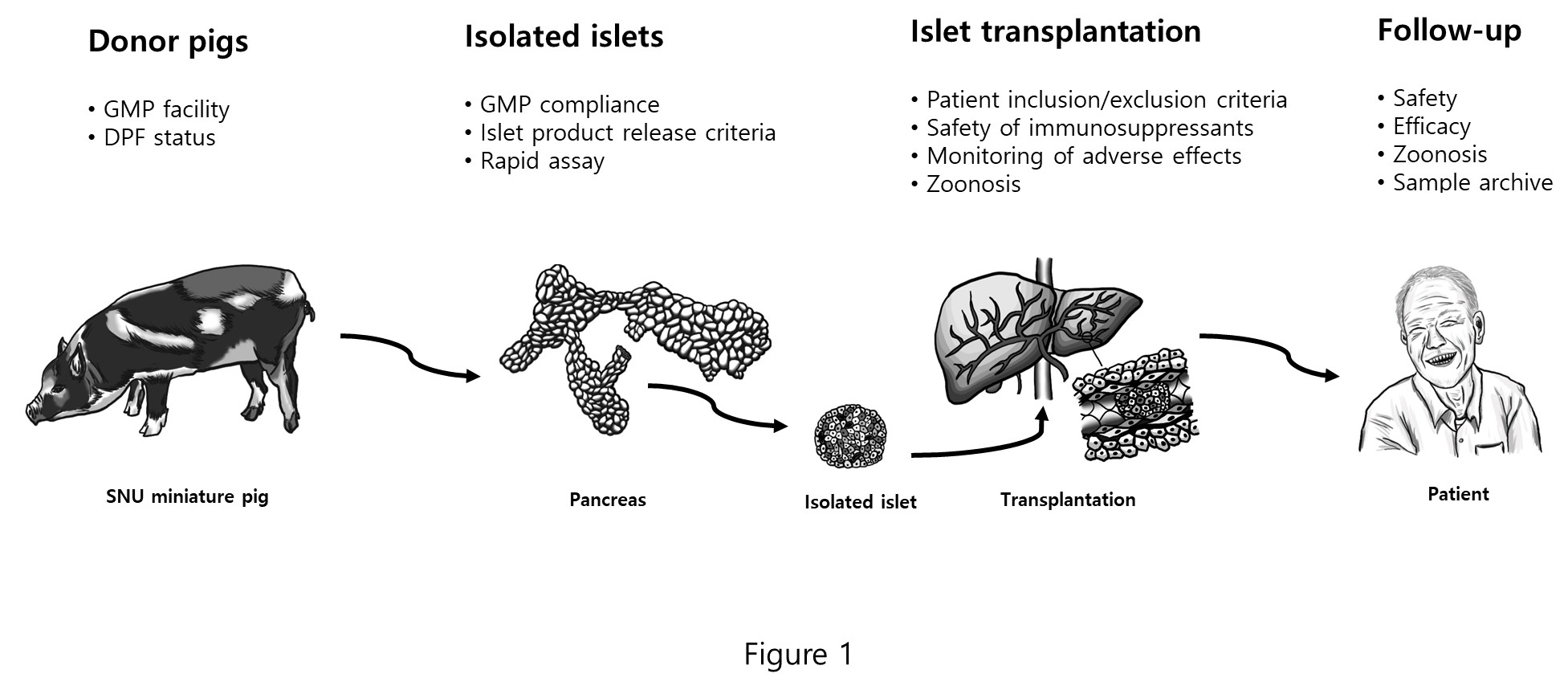
the Korea Healthcare Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry for Health and Welfare, Republic of Korea (Grant No. HI13C0954).