How to deliver a therapeutic dose of encapsulated human islets?
Rosy Eloy1, Ouardane Jouannot1, Alexandre Geissler1, Anne-Lise Gaffuri1, Maud Fumex1, Xavier Gaume1, Camille Gautier1, Yann Courbebaisse1, Karim Bouzakri2, Olivier Soula1.
1Adocia, Lyon, France; 2CEED, Strasbourg, France
Introduction: Islet transplantation is an effective cure for T1D but the requirement for immunosuppression limits its application. AdoShell® Islets, an ultrathin encapsulation device, was designed to deliver a therapeutic dose of pancreatic islets into the peritoneal cavity, provide humoral and cellular immunoisolation, and ensure retrievability. Efficacy, tolerance, and retrievability were shown in diabetic rats. Development of a human-scale implant was pursued by studying islet density, implant size and implantation in domestic pig by laparoscopy.
Methods: AdoShell® Islet composition: The composite implant consists in a suturable mechanical support and a synthetic hydrogel embedding islets.
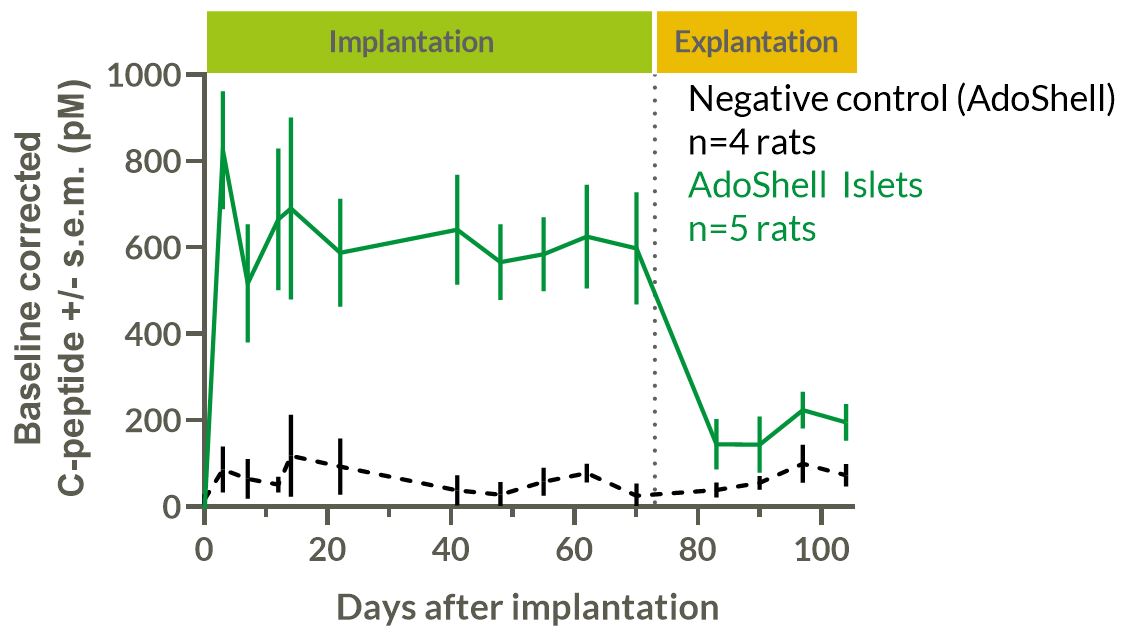
Efficacy: Diabetes was induced in outbred Wistar rats by injection of 75mg/kg streptozotocin. Wistar rat islets were encapsulated at 21kIEQ/mL in 1300µm-thick implants. 6 implants per rat were implanted for a mean dose of 20kIEQ/kg in intraperitoneal and intramuscular sites. C-peptide and glycemia were measured. Rats were explanted after 73 days and monitored for 30 extra days. Explants were analyzed in vitro by GSIS and histology.
Density/Thickness: Human islets were encapsulated at densities from 20 to 100 kIEQ/mL in AdoShell® Islets in 5mm-diameter 600µm- or 1600µm-thick disks. Their functionality was evaluated using perifusion up to 1 month.
Results: Efficacy: STZ rats having received AdoShell® Islets showed sustained 627pM baseline-corrected C-peptide secretion for 73 days. The transplanted rats showed normal levels of fasting glycemia. After implant removal, they reverted to diabetic phenotype (FIG1). Implants were well tolerated, without foreign body reaction, nor immune cell infiltration, and still secreted insulin after explantation.
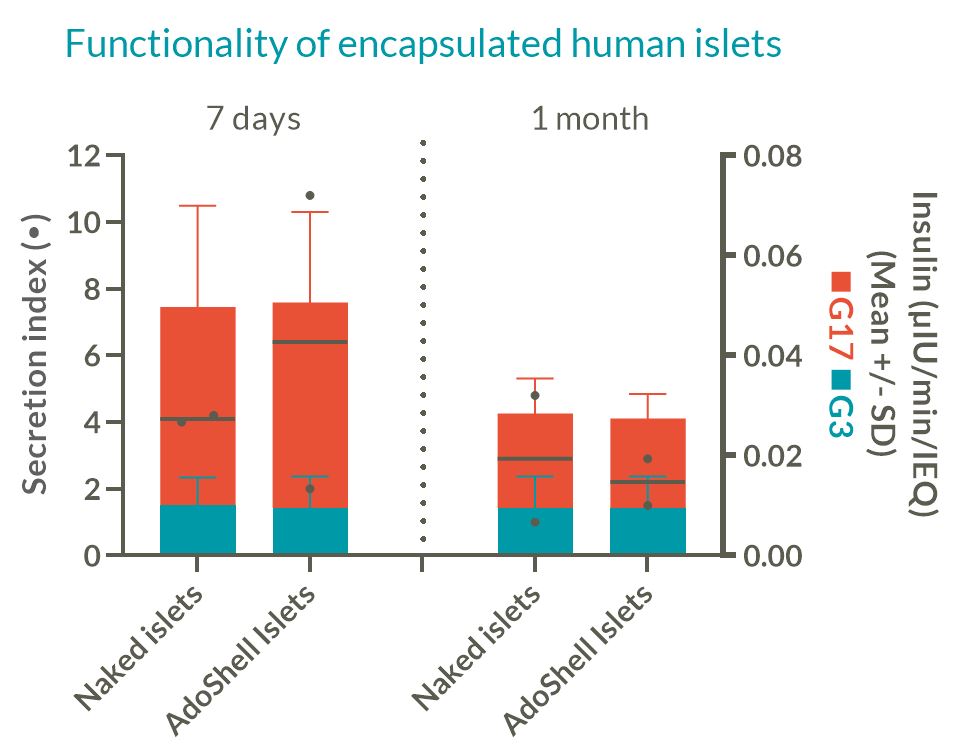
Density: High islet density leads to competition for oxygen, while low density leads to unpractical implant volumes for therapeutic doses. In vitro encapsulated human islets at 20kIEQ/mL displayed functionality comparable to naked islets in perifusion experiments up to 1 month (FIG2), whereas 50k-100kIEQ/mL functionality was reduced.
Thickness: Thickness could impact islet survival and diffusion kinetics. In vitro, encapsulated human islets were functional in both 1600µm- and 600µm-thick implants. However, it showed a lag-time of ~15 min at 1600µm versus naked islets, reduced to ~5 min at 630µm.
Implantability: A 4.4 cm-diameter 900µm-thick reinforced implant was easily implanted in retroperitoneum in domestic pig by laparoscopy . A 13cm-diameter implant capable of delivering 180kIEQ was manufactured to illustrate the scalability for clinical use.
Conclusion: Efficacy, tolerance, and retrievability were demonstrated in diabetic rats. AdoShell® Islets constitutes a retrievable implant capable of delivering a therapeutic dose in humans. It is implantable by laparoscopy and circumvents the need for life-long immunosuppressants, offering the hope to broaden the eligible T1D patient population.