Development, optimization, and preclinical evaluation of a bio-artificial pancreas for the treatment of type 1 diabetes (T1D)
Sawsen Bekir1, Mathilde Mosser1, Grégoire Mignot1, Jean-Marie Bach1.
1IECM (USC 1383/Oniris), Nantes-Atlantic National College of Veterinary Medicine, Food Science and Engineering (Oniris), Nantes, France
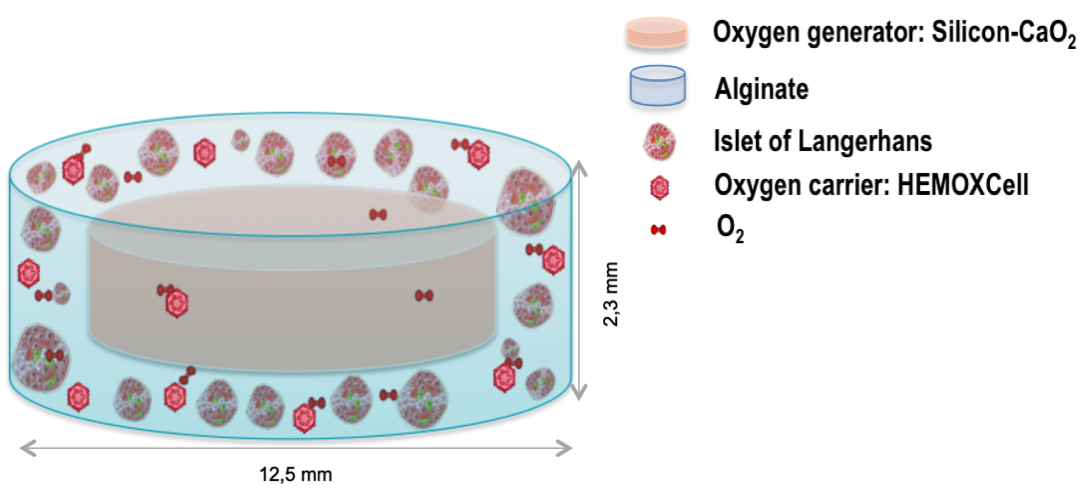
Introduction: Pancreatic islet or pancreas allotransplantation is a clinical reality for type 1 diabetes (T1D). However, this approach requires life-long immunosuppressive treatment and is limited by the shortage of available organs. The bio-artificial pancreas (BAP) encapsulating pig or human pancreatic islets in an immunoprotective matrix is a promising complementary therapy, providing a better metabolic control to manage glycaemia and overcome organ shortage. Despite key progress made so far, the main hurdle hindering the development of a clinically efficient BAP is the lack of oxygen (O2) faced by the cells during the post-transplantation two-week period needed for the BAP neovascularization. To counteract hypoxia, we developed a BAP integrating an innovative strategy of oxygenation (ISO) based on the combination of an O2 generator and an O2 carrier1.
Method: The strategy developed consists of performing a minimally invasive subcutaneous transplantation of pancreatic islets of human origin (allogeneic) or porcine xenogeneic, macro-encapsulated in a 3D alginate matrix. The device must be of a reasonable size to be transplantable in the clinic. Therefore, the configuration of the developed prototype has been optimized using the methodology of experimental design, ensuring balanced O2 availability to a very high density of islets. The biocompatibility and the efficacy of the BAP-ISO were then assessed using appropriate mice models.
Results: The BAP-ISO prototype was optimized by determining its optimal configuration and in vitro validation2. The optimal configuration of the prototype tested here is suitable for transplantation in mice and could be efficiently extrapolated to Human. The results show the safety of BAP-ISO in two mouse models. No aggressive tissue reaction or inflammation was detected macroscopically or microscopically in the surrounding tissues or in the explanted device. BAP-ISO is easily removable end with a very high islet density of murine pseudo-islets corrects diabetes earlier in transplanted mice compared to a BAP without ISO. BAP-ISO carrying neonatal porcine islets keeps them functional long-term in mice (C-peptide). Finally, BAP-ISO loaded with human islets effectively reduces basal blood sugar levels in mice, improving diabetes. Our results confirm that our prototype is biocompatible in the tested models and validates its ability to correct or improve hyperglycemia in mice.
Conclusion: The apparently positive risk/benefit ratio should allow us to propose a phase I/II clinical trial evaluating the BAP-ISO with human allogeneic islets for brittle T1D patients. Additionally, this study paves the way for the development of a BAP-ISO with porcine islets as a complement/alternative to allogeneic islets, addressing the shortage of human organs. The project's promising results could lead to significant developments in diabetes medical research and xenotherapy.
Fondation pour la recherche médicale (FRM). Agence de biomédecine. Société francophone de transplantation (SFT).
[1] Mouré, A. et al. Extracellular hemoglobin combined with an O2‐generating material overcomes O 2 limitation in the bioartificial pancreas. Biotechnol. Bioeng. 116, 1176–1189 (2019).
[2] Mouré, A. et al. Optimization of an O2-balanced bioartificial pancreas for type 1 diabetes using statistical design of experiment. Sci. Rep. 12, 4681 (2022).