Development of a model to fabricate vascularised 3D bioprinted constructs to support islet transplantation for type 1 diabetes
Anna Kulaga1, Zhilian Yue1, Xiao ` Liu1, Gordon Wallace1, Toby Coates2,3, Chris Drogemuller2,3.
1University of Adelaide, Adelaide, Australia; 2University of Wollongong, Wollongong, Australia; 3Royal Adelaide Hospital, Adelaide, Australia
Introduction: Conventional pancreatic islet transplantation is a promising treatment for individuals with severe type 1 diabetes, however 70% of islets lose function limiting the boarder application of this treatment. Islets are highly vascularised and metabolically active cluster of cells for fine tuning of blood sugar levels to achieve euglycemia. Thus, revascularisation of transplanted islets is a key focus to encourage maximal function and islet survivability. Compared to conventional islet transplantation, 3D bioprinting introduces a method for the revascularisation of islets to support their function and survivability post-transplantation by mimicking the cellular architecture found in nature. Herein we reported a novel bioprinting platform that enables endothelial cells to self-assemble to form interconnected 3D vascular networks.
Method: In this study, a colinear co-extrusion approach is utilised for the biofabrication of vascularised cell-laden constructs via a Janus nozzle which enables simultaneous side-by-side deposition of both cell-free structural ink and cell-laden bioink. The structural ink is comprised of 2% alginate-methacrylate (Alg-ma)/ 2%gelatin/7.5%gelatin methacryloyl (GelMA) and the cell-laden ink is comprised of 20mM CaCl2/2% gelatin/ 0.5mg/ml fibrinogen bioink which carries human umbilical vein endothelial cells (HUVECs). The 3D REDI a mechanical extruder 3D printer developed by TRICEP was used to fabricate a 10x10mm 4-layered scaffolds at a 300 mm/min speed, flowrate of 0.0005670 ml/min and printed at a 45° angle with a 560 µm Janus nozzle. Rheology was conducted on the structural bioink and mechanical testing was used on cell-free scaffolds to determine the physiochemical properties of the structural bioink and mechanical properties of the scaffold. To evaluate the cellular viability and function, observation of cellular behaviour, live/dead staining, and immunostaining for CD31 were conducted.
Results: Rheological characterization demonstrated shear thinning behavior but also self-recovery behavior upon shear rate whilst mechanical testing supported structural stability of the construct. 3D bioprinting of HUVEC-laden constructs presented viable cells post-printing up to day 28 represented through live/dead staining but also cell morphological changes on day 1 and day 7.
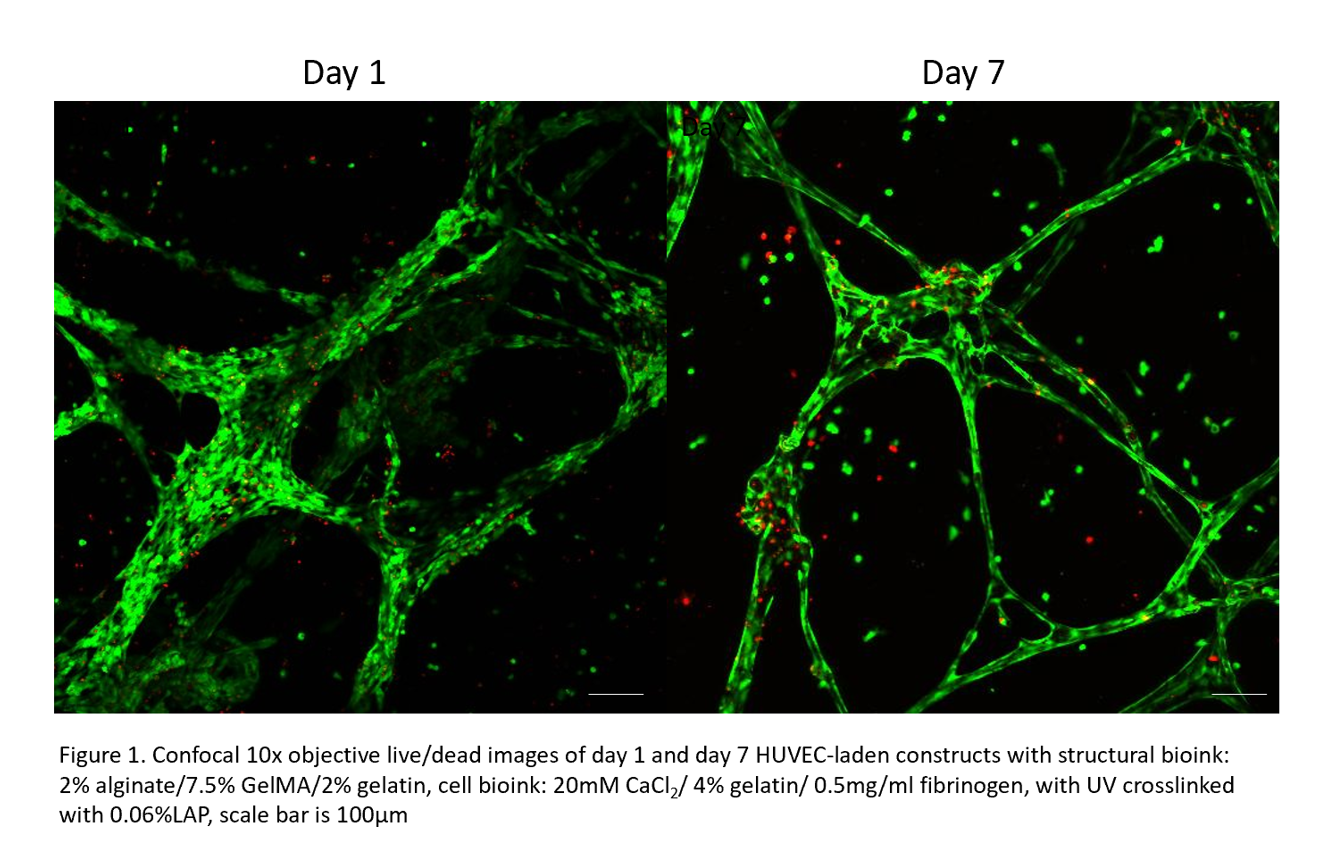
HUVECs were able to self-assemble into interconnected vessel like formations throughout the whole construct and expressed the CD31 marker.
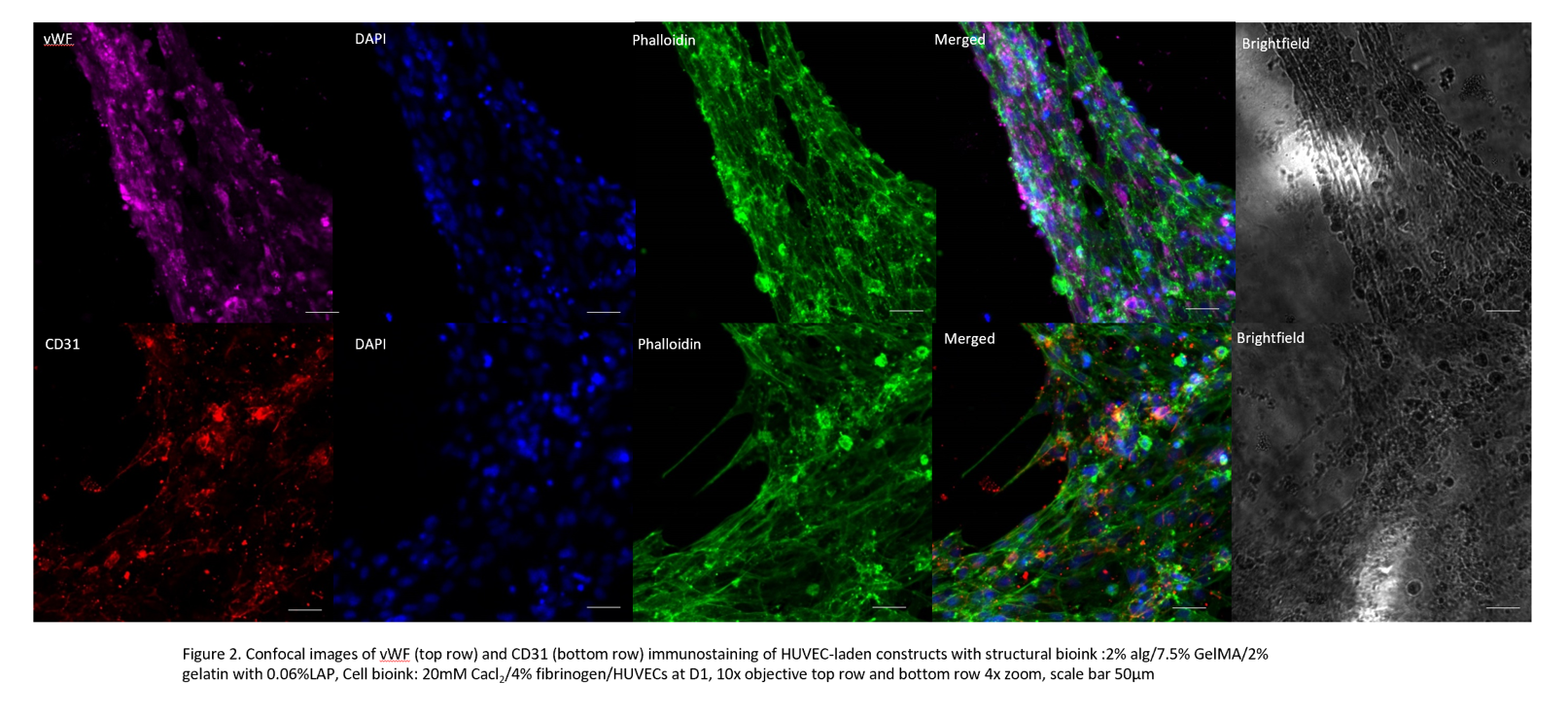
Conclusion: Colinear bioprinting precisely deposits filaments aiding structural integrity but also the formation of interconnected channels within the construct making this a highly attractive method for vascularisation. The ongoing in vivo study will provide further insight into how to optimise the colinear printing platform to improve vascularisation and islet survival rate following islet transplantation.