Gelatin methacrylate and polyethylene glycol diacrylate 3D printed hydrogels support vascularization for islet transplantation
Martha Fowler1, Boram Kim1, Alvaro Moreno Lozano1, Shalini Pandey1, Joe Swain2, Jeffrey Hartgerink2, Omid Veiseh1.
1Bioengineering, Rice University, Houston, TX, United States; 2Chemistry, Rice University, Houston, TX, United States
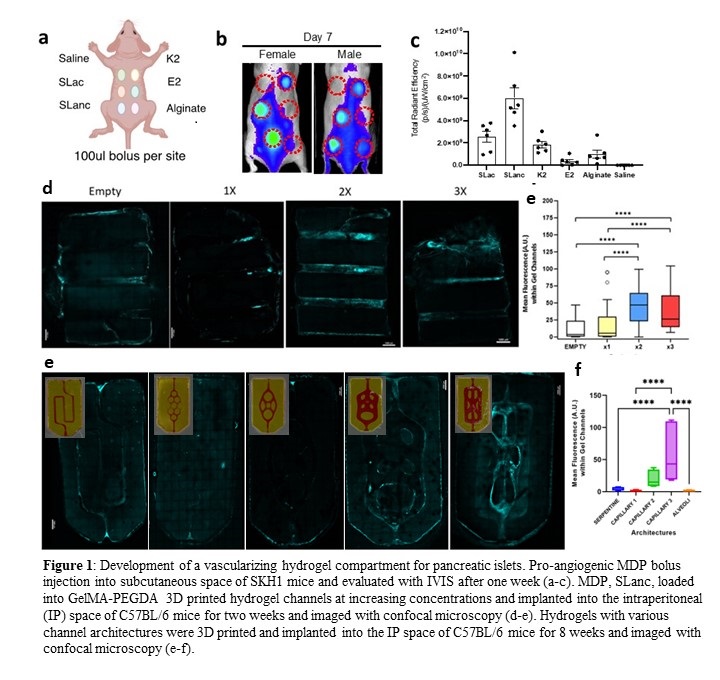
Type 1 Diabetes (T1D) is a chronic, autoimmune disease of the pancreas. In T1D, insulin-producing cells, known as islet beta cells, are destroyed by the host immune system causing lifelong regulation of patient glucose levels. Islet replacement therapy is a promising treatment for T1D. However, transplanted islets are targeted by the host immune system, have poor cell survival and retrieval, and require lifelong immunosuppression. Alternatively, islets can be encapsulated in macro devices composed of biomaterials that evade the immune system and allow local implantation and retrieval. Islet survival remains a key challenge in macro devices due to poor nutrient diffusion. 3D-printed gelatin methacrylate (GelMa) and polyethylene glycol diacrylate (PEGDA) hydrogels can be fabricated with open channels to encourage vascular ingrowth into 3D constructs. Multidomain peptide (MDP) VEGF-mimic hydrogels have been shown to improve angiogenesis in vivo and can be utilized as a pro-angiogenic agent within hydrogel channels. We hypothesized that an open-channel hydrogel with MDP will promote vascularization to nearby encapsulated islets and long-term islet survival. In vivo fluorescence imaging was performed to evaluate various pro-angiogenic peptide injections in subcutaneous space of SKH1 mice to determine the lead. We selected SLanc as it exhibited the best proangiogenic potential in vivo (Fig. 1 a-c). A 10wt% GelMA and 3.25wt% 3.4 kDa PEGDA hydrogel was printed with a LumenX stereolithography printer. MDP was loaded into hydrogel channels at various concentrations prior to implantation. MDP loaded hydrogels were implanted into the fat pads of 6-10 week old C57BL/6 male mice. Mice were intravenously injected with fluorescent dextran and explanted after 2 weeks. Samples were evaluated with confocal microscopy. We found that MDP-loaded hydrogels demonstrated vascular formation after 2 weeks of fat pad implantation in-vivo with MDP concentrations above 2X (Fig. 1d-e). Secondly, the hydrogels were 3D printed to possess different channel architectures and implanted as described above. After 8 weeks, hydrogels were retrieved and analyzed. We found that larger, more open architectures encourage host vessel infiltration (Fig. 1f-g). Our work demonstrates promising formulations and hydrogel structures for establishing vascularization within an implantable construct for T1D therapeutic applications. The pro-angiogenic peptide formulation demonstrates that vascularization can be achieved through a peptide formulation and without immunosuppressants. MDP formulations will be further assessed with the addition of an encapsulated islet compartment that is surrounded by our vascularizing 3D-printed hydrogel with the most pro-angiogenic architecture to promote nutrient delivery to islets. Islet functionality and glycemic correction will be measured to confirm the significance of the vascular hydrogel compartment and our vascular formulations.
Juvenile diabetes research foundation (JDRF), National Institute of Health (NIH), Defense Advanced Research Projects Agency (DARPA), Lilie Innovation Fellowship.
[1] A. Vegas and O.Veiseh et al. Nat Biotechnol. 34 (2016) 345-52
[2] A. Vegas and O. Veiseh et al. Nat Med. 22 (2016) 306-11
[3] M. Bochenek et al. Nat Biomed Eng. 2 (2018) 810-2
[4] Shea and Desai, Nat Rev Drug Discov., 2017
[5] Vaithilingam et al., Rev Diabet Stud., 2017
[6] B. Grigoryan et al, Science 2019
[7] M, Royse et al. Biomater Sci. 10 (2022)
[8] S. Parkhideh et al. Biomater Sci. 11 (2023)
[9] NC, Carrejo et al. ACS Biomater Sci Eng. 4 (2018) 1386-96