Modifying The Porcine Genome for Xeno-organ Transplantation
Juan Fernández1, Björn Petersen1, Antje Frenzel1, Petra Hazel1, Andrea Lucas-Hahn1, Paul Kielau1.
1Institute of Farm Animal Genetics (ING), Friedrich-Loeffler-Institut, Neustadt Am Rübenberge, Germany
Department of Biotechnology.
Background: The immunological incomptatibility between pig and human organs, which leads to hyperacute, acute vascular and cellular rejection is still an important barrier that needs to be surmounted. Moreover, genetically modified pigs are mostly generated by microinjection or somatic cell nuclear transfer (SCNT). Both of these techniques are difficult to handle and may also cause embryo damage due to their invasiveness.
For this purpose, the points discussed in Study A and Study B attempt to give rise to possible solutions and alternatives to these impediments.
Aims:
Study A: Introduction of CRISPR/Cas9 into in vitro fertilized (IVF) porcine zygotes by electroporation which might be an easier and less harmful technique to introduce endonuclease systems into embryos
Study B: Introduction of four human transgenes and simultaneous knock out of the swine xenoantigen GGTA1 in gene-edited (5xTG/GGTA1+/-) pig fibroblast cells to achieve xenograft protection.
Methods
Study A: The fluorescent compound TMR-Dextran was first introduced into parthogenetically activated pig zygotes to test and establish optimal parameters for the electroporation procedure. The resulting protocol was then implemented for the electroporation of GGTA1,CMAH and B4GAL crRNAs/Cas9 complexes into IVF embryos. Dextran electroporation into IVF embryos was also performed as a positive control.
Study B: A vector encoding the four human transgenes Thrombomodulin (hTHBD), CD47 and the protein complex B2M/HLA-E was introduced into genetically edited pig fibroblasts, that are heterozygous for GGTA1 and express five proteins that avoid complement system activation, using a PiggyBac-mediated transfection approach. Additionally, a vector targeting GGTA1 for knock-out was simultaneously introduced. To select cells that are negative for GGTA1, a streptaividin-biotin affinity assay was utilized. These cells were serially diluted to select cell colonies that are positive for the transfected transgenes to evaluate them for SCNT.
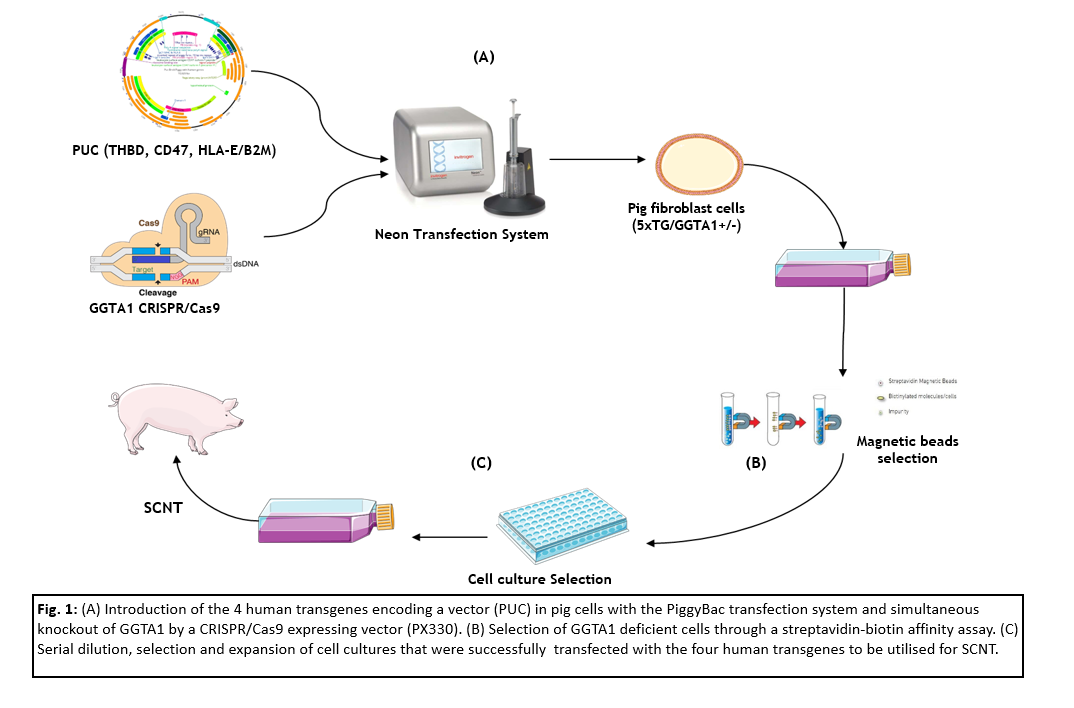
Results
Study A
The introduction of the red fluorescent protein TMR-Dextran into parthogenetically activated pig zygotes was successfully achieved yielding a blastocyst formation range between 30 and 60 percent. Electroporation parameters were applied for the introduction of the CRISPR/CAS9 System into IVF zygotes for the disruption of the xenoantigenes CMAH, B4GAL and GGTA1
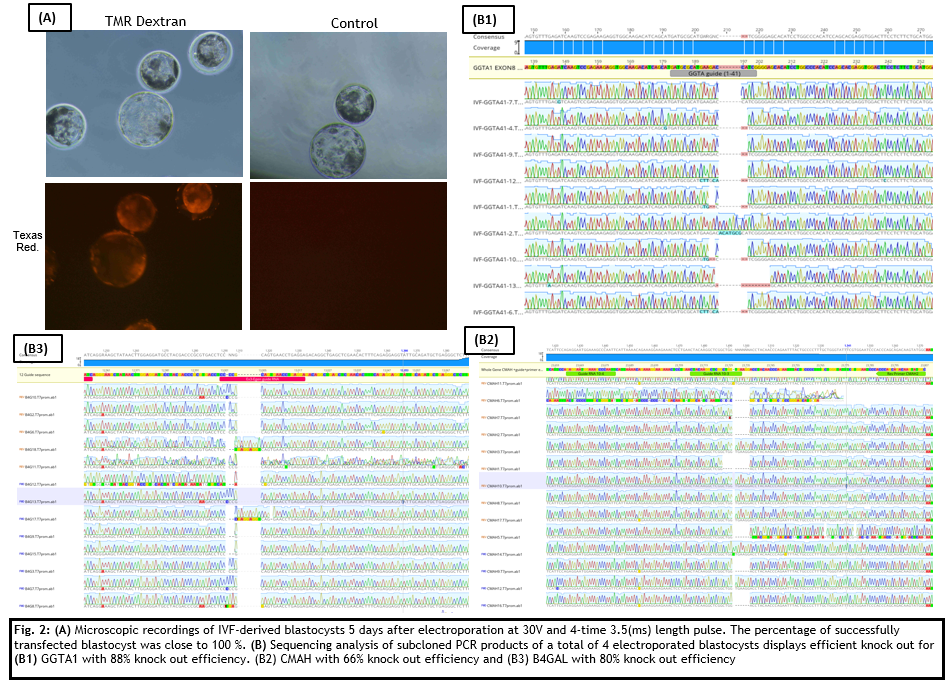
Study B
Double knockout of GGTA1 and genome insertion of hTHBD, hCD47 and B2M/HLA-E protein complex in (5xTG/GGTA1+/-) pig fibroblast cells was successfully achieved . mRNA expression was also analyzed for the different fibroblast cell cultures, demonstrating effective efficiency of this approach.
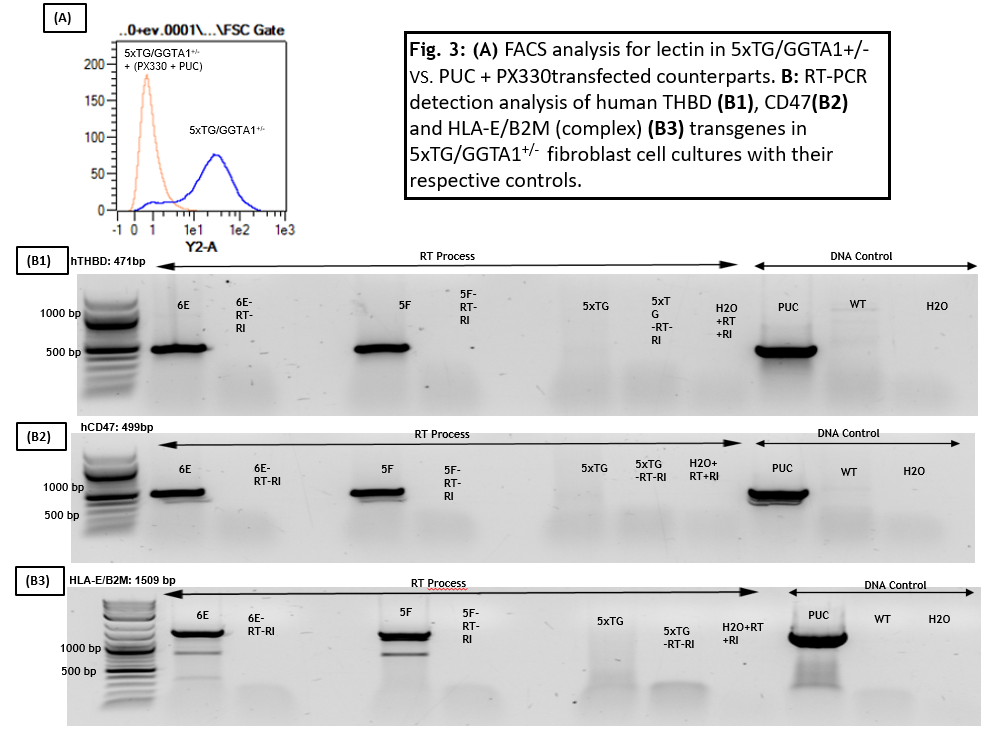
Conclusion: In both of these studies gene-editing tools were successfully delivered in pig cells to further modify their genome. For both studies, the creation of genetically-edited pigs is desired to analyze their potential as organ donors.
The project is funded by the German Research Foundation (DFG). BU 3523/1-1, OH 166/2-1, KO 3552/8-1.. Smart Servier Medical Art (SMART - Servier Medical ART ).
[1] Murphy, K., Travers, P., Walport, M., & Janeway, C. (2018). Janeway's immunobiology. New York: Garland Science; Int. J. Mol. Sci.2021, 22(5), 2249
[2] Petersen, B., Frenzel, A., Lucas-Hahn, A., Herrmann, D., Hassel, P., Klein, S., Ziegler, M., Hadeler, K.-G. and Niemann, H. (2016), Efficient production of biallelic GGTA1 knockout pigs by cytoplasmic microinjection of CRISPR/Cas9 into zygotes, Xenotransplantation, 23: 338– 346. doi: 10.1111/xen.12258
[3] Petersen B, Ramackers W, Tiede A, Lucas-Hahn A, Herrmann D, Barg-Kues B, Schuettler W, Friedrich L, Schwinzer R, Winkler M, Niemann H. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation. 2009 Nov-Dec;16(6):486-95. doi: 10.1111/j.1399-3089.2009.00537.x. PMID: 20042048.
[4] Tanihara F, Hirata M, Nguyen NT, Sawamoto O, Kikuchi T, Otoi T. One-Step Generation of Multiple Gene-Edited Pigs by Electroporation of the CRISPR/Cas9 System into Zygotes to Reduce Xenoantigen Biosynthesis. Int J Mol Sci. 2021 Feb 24;22(5):2249. doi: 10.3390/ijms22052249. PMID: 33668187; PMCID: PMC7956194