Braulio A Marfil-Garza, Canada has been granted the IPITA Congress Scientific Award

BETA-2 scores overestimate graft function in pancreatic islet transplant recipients with an estimated glomerular filtration rate of <30 ml/min/1.73m2
Braulio Marfil-Garza1,2,3, Alice LJ Carr1,2, Meiying Zhuang4, Anna Lam1,2, Khaled Dajani1,2, Blaire Anderson1,2, Doug O'Gorman1,2, Tatsuya Kin1,2, David Bigam1, James Shapiro1,2, Peter A Senior1,2.
1Clinical Islet Transplant Program, University of Alberta, Edmonton, AB, Canada; 2Alberta Diabetes Institute, Alberta Diabetes Institute, Edmonton, AB, Canada; 3Excellence and Innovation Center - Translational Research Unit, CHRISTUS LatAm Hub, Monterrey, , Mexico; 4Department of Medicine - Division of Endocrinology, University of British Columbia, Vancouver, BC, Canada
Introduction: The BETA-2 score is a clinical composite score used to assess graft function after islet transplantation (ITx). This score incorporates C-peptide, fasting plasma glucose, hemoglobin A1c, and insulin requirements. However, C-peptide undergoes renal clearance and may accumulate in people with renal impairment. We sought to examine the effect of renal impairment on the BETA-2 score in ITx recipients.
Methods: Cross-sectional study of ITx recipients at the University of Alberta. Most recent BETA-2 scores (pre-graft failure), and its components (insulin requirements, fasting plasma glucose, HbA1c and serum C-peptide), as well as eGFR during BETA-2 assessment (CKD-EPI) were obtained. Analyses were stratified by insulin dependence status and eGFR groups (using KDIGO stages of CKD). Medians and interquartile ranges (IQR) are presented, statistical comparisons were made using chi-square, Wilcoxon rank sum and Kruskal-Wallis tests, as appropriate. Segmented regression was used to identify inflection points where the BETA-2 score was disproportionally affected by changes in eGFR.
Results: We include 178 recipients (42% male, median (IQR) age at first transplant of 52.3 (44.8-58.6) years) who received a median of 2 (2-3) islet infusions and 15,312 (11,705-20,278) islet equivalents/kg. Median BETA-2 scores were 11.1 (6.0-19.4), with a median time from first transplant to BETA-2 score calculation of 8.0 (4.2-11.9) years. A total of 37% of recipients were insulin independent, with similar rates observed between eGFR groups (p=0.631).
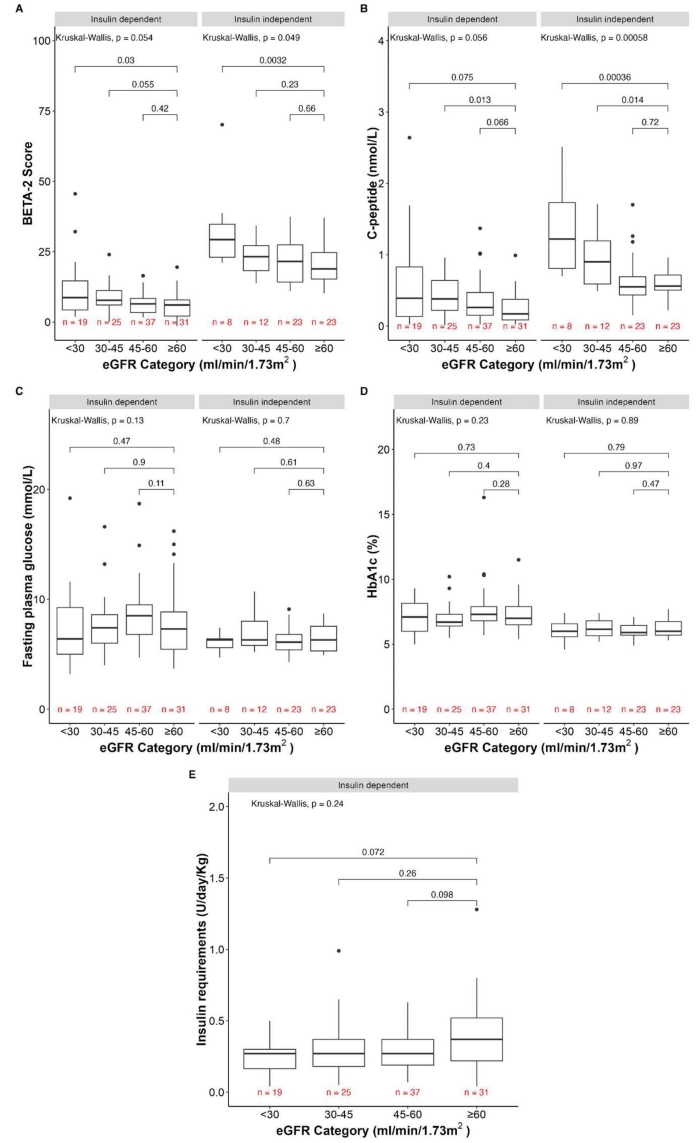
Overall, BETA-2 scores were higher in ITx recipients with stage 4 CKD (<30 ml/min/1.73m2) compared to stage 1-2 CKD (>60 ml/min/1.73m2) for those being insulin dependent (p=0.03) and insulin independent (p=0.0032) (Figure 1A). These differences were driven by C-peptide levels, which were increased in recipients with stage 4 CKD compared to stage 1-2 CKD in those being insulin dependent (p=0.075), but more importantly in those being insulin independent (p=0.00036) (Figure 1B). All other BETA-2 score components were similar between groups (Figure 1C-E).
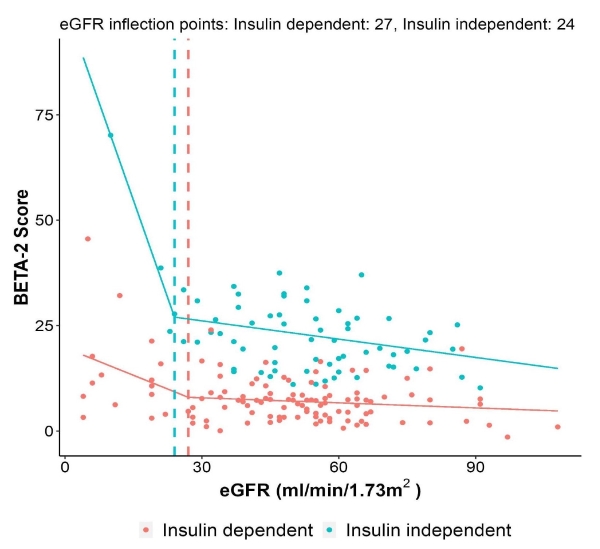
Segmented linear regression of the BETA-2 score with eGFR demonstrated an inflection point of eGFR 27 ml/min/1.73m2 for recipients being insulin dependent and 24 ml/min/1.73m2 for those being insulin independent (Figure 2).
Conclusions: This study shows that BETA-2 scores increase as eGFR declines, driven by the C-peptide component of the score, a phenomenon that is most notable in insulin independent recipients. These higher C-peptide levels (and BETA-2 scores) do not reflect better graft function, as they are not associated with better glycemic control or higher insulin independence rates. Our data suggests that in recipients with eGFR<30 ml/min/1.73m2, BETA-2 scores and C-peptide levels should be cautiously interpreted.
The Clinical Islet Transplant Program is funded by the Government of Alberta, the Juvenile Diabetes Research Foundation, the US National Institute of Diabetes and Digestive and Kidney Diseases, the Diabetes Research Institute Foundation of Canada, and the Immune Tolerance Network. JS holds a Canada Research Chair (Tier 1) in Regenerative Medicine and Transplantation Surgery, and is supported through grant support from the Juvenile Diabetes Research Foundation, Diabetes Canada, The Canadian Donation and Transplant Research Project, the Diabetes Research Institute Foundation of Canada (DRIFCan), the Alberta Diabetes Foundation, and the Canadian Stem Cell Network. BM-G is supported by an Alberta Transplant Institute scholarship and the CHRISTUS Excellence and Innovation Center. AL and PAS are supported by the Alberta Academic Medicine Health Services Program. PAS holds the Charles A Allard Chair in Diabetes Research. We acknowledge a large group directly or indirectly involved in the clinical care of patients undergoing pancreatic islet transplantation at the University of Alberta (Edmonton, AB, Canada), including Edmond Ryan, Norman Kneteman, Breay W Paty, and Angela Koh, as well as surgical fellows, technicians, and support staff from the Clinical Islet Transplant and Human Organ Procurement and Exchange Programs..