Stable graft function and glycemic control 6 years after clinical islet transplantation on the omentum: A case report
David Baidal1,2, Ana M Alvarez1, Camillo Ricordi1, Rodolfo Alejandro1,2.
1Diabetes Research Institute, University of Miami, Miami, FL, United States; 2Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, Miller School of Medicine, University of Miami, Miami, FL, United States
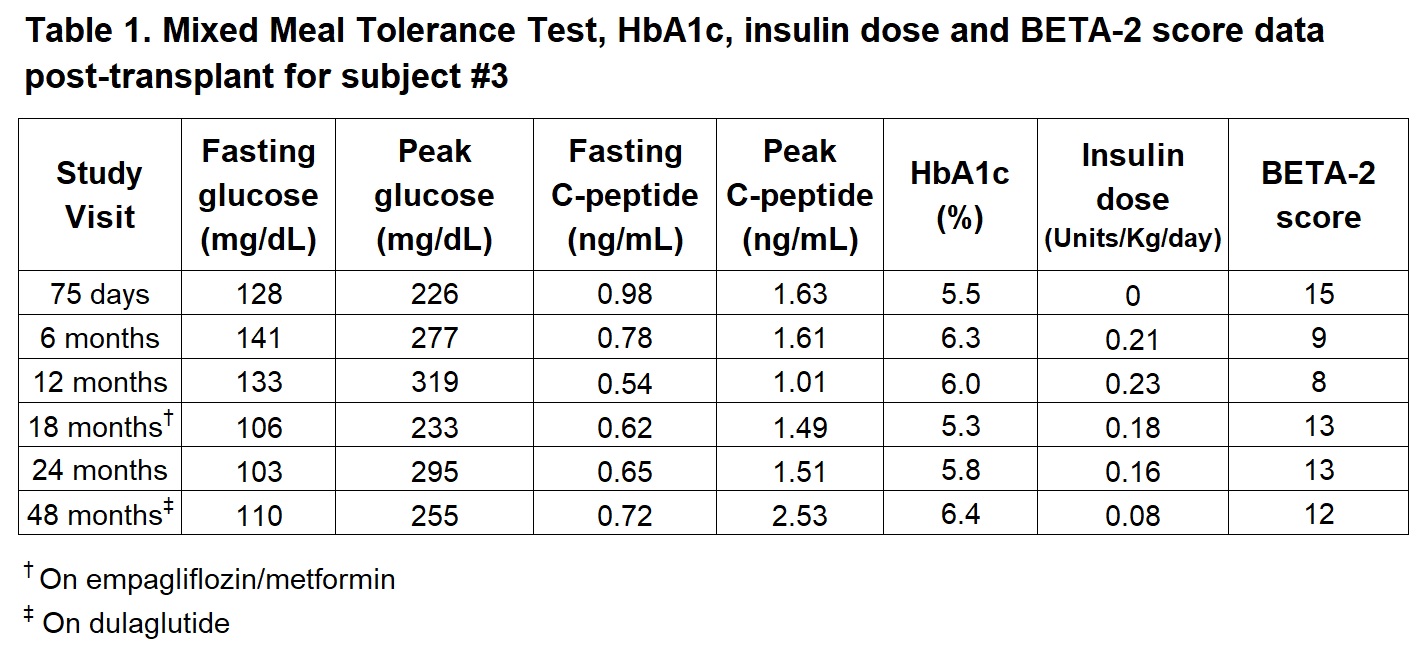
Introduction: We have previously shown safety and efficacy of islet transplantation (ITx) in the omentum in 3 subjects with type 1 diabetes (T1D). Two subjects (#1 and #3) achieved the primary outcome at 1-year (A1c ≤6.5%; No severe hypoglycemia). Subject 1 developed progressive decline in C-peptide after 15 months likely attributed to change in immunosuppression. We report on 6-year follow-up data for subject #3.
Methods: A 46 y/o woman with T1D of 26 years duration underwent single ITx on the omentum (12,648 IEQ/Kg) using a biologic scaffold under ATG induction and mycophenolate sodium + tacrolimus maintenance immunosuppression (IS). Exenatide extended-release (Bydureon®) 2 mg subcutaneously once weekly was started on post-operative day -2 but discontinued after 4 doses due to side effects. At 46-months, maintenance IS was changed to azathioprine and tacrolimus XR (Envarsus XR®). Pre-ITx insulin total daily dose (TDD) was 0.45 units/Kg/day and HbA1c 6.3%. Mixed meal tolerance test (MMTT), HbA1c and insulin requirements were obtained at follow-up visits up to 4 years post-ITx. Continuous glucose monitoring (CGM) metrics and insulin requirements were obtained at 6 years post-ITx. To improve and maintain adequate glycemic control, combination empagliflozin/metformin was added after the 12-month visit and stopped 8-months later due to associated side effects. Dulaglutide (Trulicity®) 0.75 mg SC weekly was introduced at 42-months post-transplant with good tolerability.
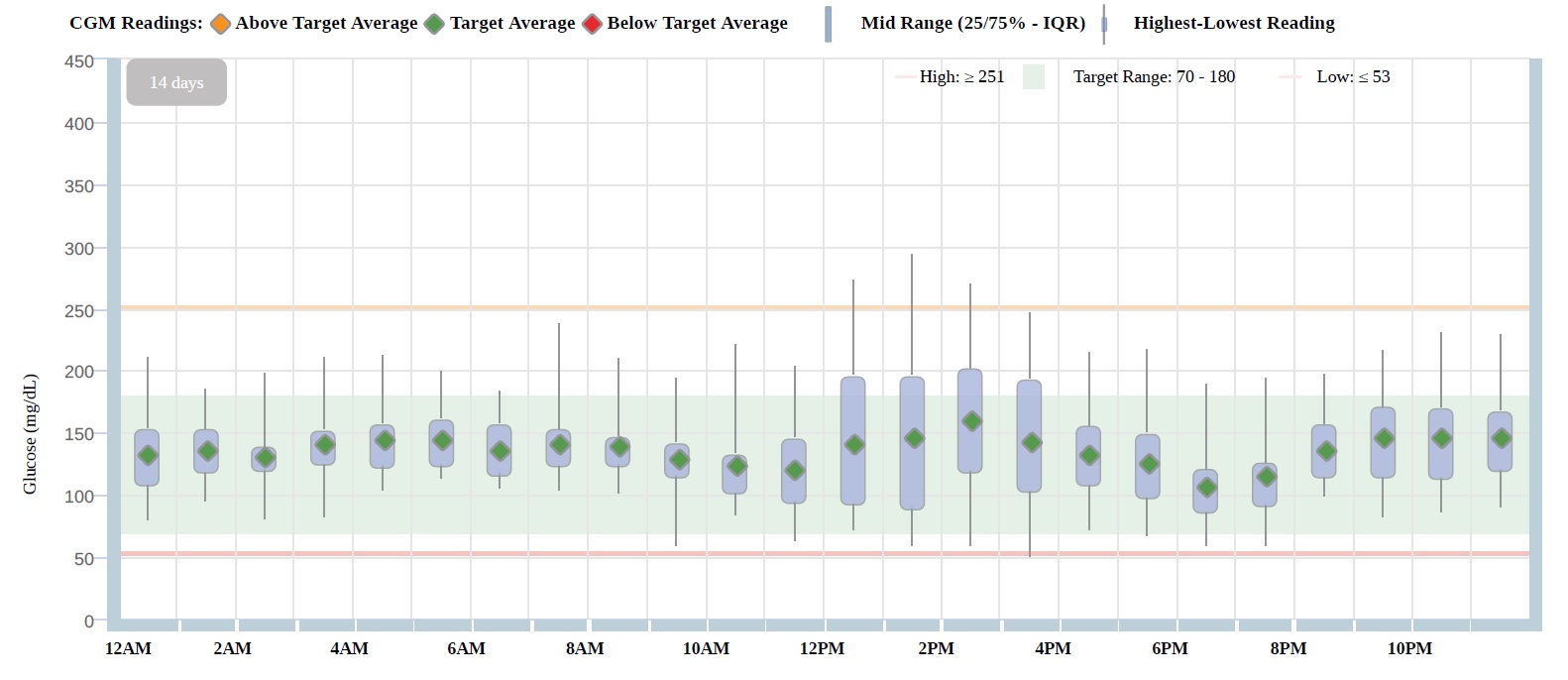
Results: At 4-years post-ITx, fasting glucose was 110 mg/dL, fasting C-peptide 0.72 mg/dL and MMTT peak C-peptide 2.53 ng/mL, with insulin TDD 0.08 units/Kg/day, HbA1c 6.4%, and BETA-2 score 12 (Table 1). At 6-years post-ITx, CGM 14 days data review shows: GMI 6.6%, time in target range (70-180 mg/dL) 86%, time below range (<70 mg/dL) 1%, time below range (<54 mg/dL) 0%, and CV 28%, with insulin TDD of 0.14 unit/Kg/day. Summary of CGM summary of values over a 14-days period is shown on Figure-1.
Conclusions: The omentum site can allow for long-term survival of engrafted islets leading to excellent metabolic control, minimal glycemic variability, and abolishment of severe hypoglycemia. Studies aimed at testing strategies to improve and sustain long-term graft survival at this novel site are needed.