Relation between primary graft function and 5-year outcomes of islet allogeneic transplantation in type 1 diabetes: a retrospective cohort study in 1210 participants from the Collaborative Islet Transplant Registry
Mikael Chetboun1, Elodie Drumez1, Cassandra Ballou2, Mehdi Maanaoui1, Elizabeth Payne2, Franca Barton2, Julie Kerr-conte1, Marie-Christine Vantyghem1, Lorenzo Piemonti3, Michael R Rickels4, Julien Labreuche1, François Pattou1.
1CHU Lille, Lille, France; 2Emmes Company, CITR, Rockville, MD, United States; 3Diabetes Research Institute, IRCCS Ospedale, San Raffaele, Milan, Italy; 4Division of Endocrinology, Diabetes & Metabolism, Hospital of the University of Pennsylvania, Philadelphia, PA, United States
CITR investigators study group.
Background: Allogeneic islet transplantation (IT) is a validated therapy in type 1 diabetes. The mechanisms underlying the decline of islet graft function with time are unclear. We evaluated the distinct relation between primary graft function (PGF) and 5-year IT outcomes.
Methods: This retrospective multi center cohort study enrolled all participants from the Collaborative Islet Transplant Registry, who received IT alone, or after kidney transplantation, between 01/19/1999, and 07/17/2020 with a calculable PGF (exposure of interest), measured 28 days after last islet infusion with a validated composite index of islet graft function (Beta2-score). Primary outcome was cumulative incidence of unsuccessful IT, defined as HbA1c ≥ 7.0% (53 mmol/mol) and/or severe hypoglycemia and/or fasting C-peptide < 0.2 ng/mL. Secondary outcomes were: 1) graft exhaustion (fasting C-peptide < 0.3 ng/mL), 2) inadequate glucose control (HbA1c ≥ 7.0% (53 mmol/mol) and/or severe hypoglycemia), and 3) requirement for exogenous insulin therapy (≥14 consecutive days). Relations between PGF and IT outcomes were explored with a competing risk analysis adjusted for all covariates suspected or known to impact outcomes. A predictive model based on PGF was built and internally validated by using bootstraps resampling method.
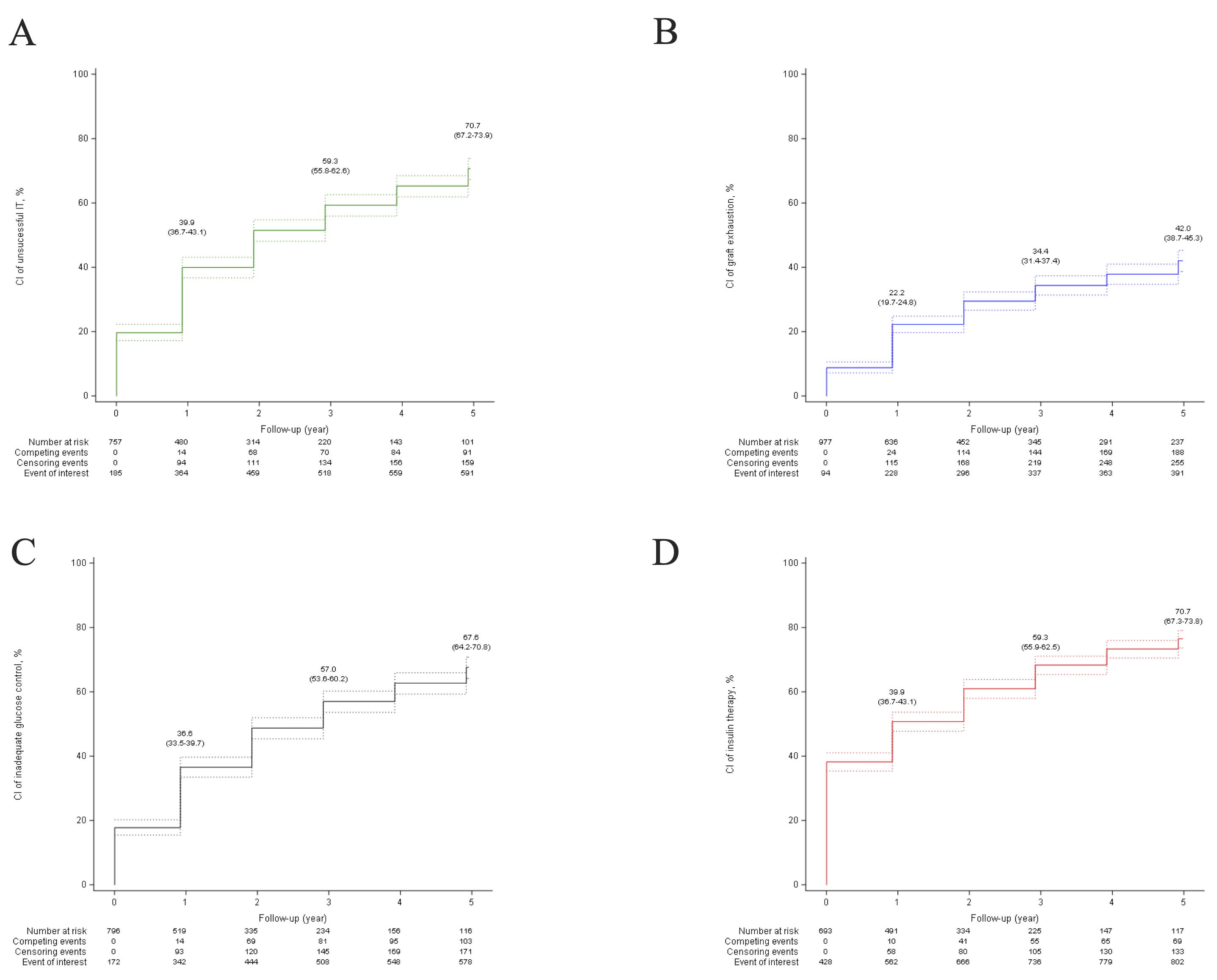
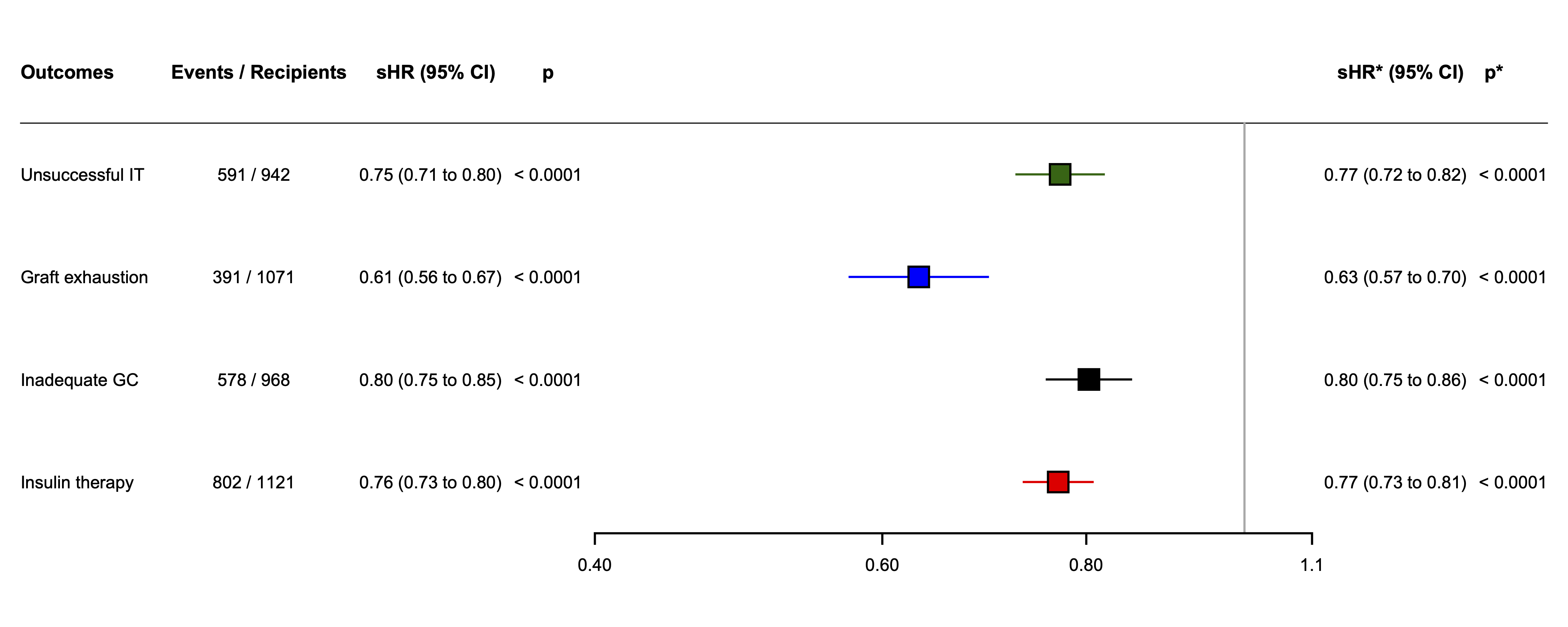
Results: In 39 centers, 1210 patients (712 (59.5%) females, mean age 47 years (SD 11) received a median of 10.8 thousand islet-equivalents per kg of bodyweight (IQR 7.4-13.5). Among them, 211 (17.6%) were islet after kidney recipient; 452 (37.4%) received a single islet infusion and 758 (62.6%) received multiple islet infusions. Mean PGF was 14.3 (SD 8.8). The 5-year cumulative incidence of unsuccessful IT was 70.7% (95%CI 67.2-73.9) (Figure 1), and was inversely and linearly related to PGF with adjusted subhazard ratio (sHR) of 0.77 (95% CI 0.72-0.82) per 5 units increase of Beta2-score (p<0.0001) (Figure 2). Secondary endpoints were similarly related to PGF. PGF predicted the 5-year cumulative incidences of unsuccessful IT, graft exhaustion, inadequate glucose control, and exogenous insulin therapy with a good accuracy with median C-statistic values of 0.70 (range 0.69-0.71); 0.76 (range 0.74-0.77); 0.65 (range 0.64-0.66); 0.72 (range 0.71-0.73), respectively. Finally, the prediction models developed from the present study were integrated into a software program allowing to display the prediction of cumulative incidences and median survival of the four IT study outcomes (https://lille-model.shinyapps.io/PGF-islet/).
Conclusion: This global multiple center study demonstrated a linear and independent relation between PGF and 5-year clinical outcomes of IT. The main study limitations are its retrospective design and the absence of analysis of complications.
Part of the results were presented at IPITA congress 2021, EPITA Congress 2023, The present study is accepted for publication in the Lancet Diabetes & Endocrinology
There was no direct funding source for the present study. The Collaborative Islet Transplant Registry is supported by Public Health Service research grants UCH DK098086 and UC4 DK114839 from the National Institutes of Health, and in the past by a supplemental grant from the Juvenile Diabetes Research Foundation International. MRR is supported in part by Public Health Service research grant R01 DK091331. Inserm U1190 was supported by grants from Agence National de la Recherche (EGID- European Genomic Institute for Diabetes, ANR-10-LABX-46), Fondation de l'Avenir, Fonds de Dotation Line Renaud -Loulou Gasté, I-Site ULNE. Authors would like to thank Pierre Bauvin for his help in setting up the online calculator, and Alain Duhamel and the Emmes company and its collaborators for the scientific, methodological and statistical interactions that have contributed to the present study..