Routine cold storage (5 hours) leads to hyperacute graft loss in pig-to-primate kidney xenotransplantation; hypothermic machine perfusion may be preferred preservation modality
Yu Hisadome1, Daniel Eisenson1, Hayato Iwase1, Michelle Santillan1, Weili Chen1, Kazuhiro Takeuchi1, Megan Sykes2, David Sachs2, Hisashi Sahara1, Andrew Cameron1, Kazuhiko Yamada1,2.
1Surgery, Johns Hopkins University, Baltimore, MD, United States; 2Columbia Center for Translational Immunology, Columbia University, New York, NY, United States
Introduction: Xenotransplantation (XTx) is an increasingly realistic solution to the organ shortage. Clinical XTx may require off-site procurement in an SPF facility which will lead to a period of cold ischemic time during transportation. The aim of this study is to assess the effect of kidney preservation strategies on early graft function in pig-to-baboon XTx.
Method: We performed 14 pig-to-baboon kidney XTx. Pig kidney grafts were either GalTKO (n=6) or GalTKO+hCD55 (n=8). 8 cases were performed with 5 hours of cold ischemic time (preservation group). Graft kidney was preserved using either static cold storage (SCS) (n=4) or a hypothermic machine perfusion (HMP) device (Lifeport) (n=4). Preservation group was compared to 6 cases of pig donor- and baboon recipient-matched XTx performed with minimal ischemic time (control group). Two cases of MHC-matched pig-to-pig kidney Tx were performed as HMP control. All recipients underwent similar immunosuppression using anti-CD40 and mycophenolate mofetil. Recipients were screened and stratified by complement dependent cytotoxicity (CDC). All recipients were followed for up to 14 days after transplantation to evaluate early graft function.
Results: In the 8 cases of pig-to-baboon xenotransplantation with cold ischemic time (preservation group), 6 transplants with low CDC (<35%) experienced hyperacute xenograft loss of SCS kidneys, but accepted kidney grafts from the same donors stored by HMP with moderate ischemia reperfusion injury (IRI) and maximum measured creatinine (cre) of 2.0 mg/dL on POD1 (Figure 1), compared to minimal IRI (mean peak cre 0.9 mg/dL) in pig donor- and recipient-matched controls. One recipient with high CDC (preservation group) hyperacutely rejected both kidneys that were reperfused simultaneously, but HMP delayed hyperacute rejection by 2 hours; by comparison, 1 pig donor- and recipient-matched control also hyperacutely rejected its xenograft. Immunofluorescence staining of a SCS kidney in the preservation group revealed C3 deposition on glomeruli despite mild IgM binding, suggesting predominant complement activation associated with ischemia-reperfusion injury (IRI). In the 2 cases of pig-to-pig kidney Tx, kidneys reperfused after 5 hours of HMP sustained IRI with cre elevation up to 5.1 mg/dL.
Conclusion: Our data indicates that porcine kidneys appear to be particularly sensitive to IRI after cold preservation, especially across xenogeneic barriers, and routine static cold storage leads to hyperacute graft loss even in recipients with low CDC. Hypothermic machine perfusion minimizes IRI and may prevent early xenograft loss.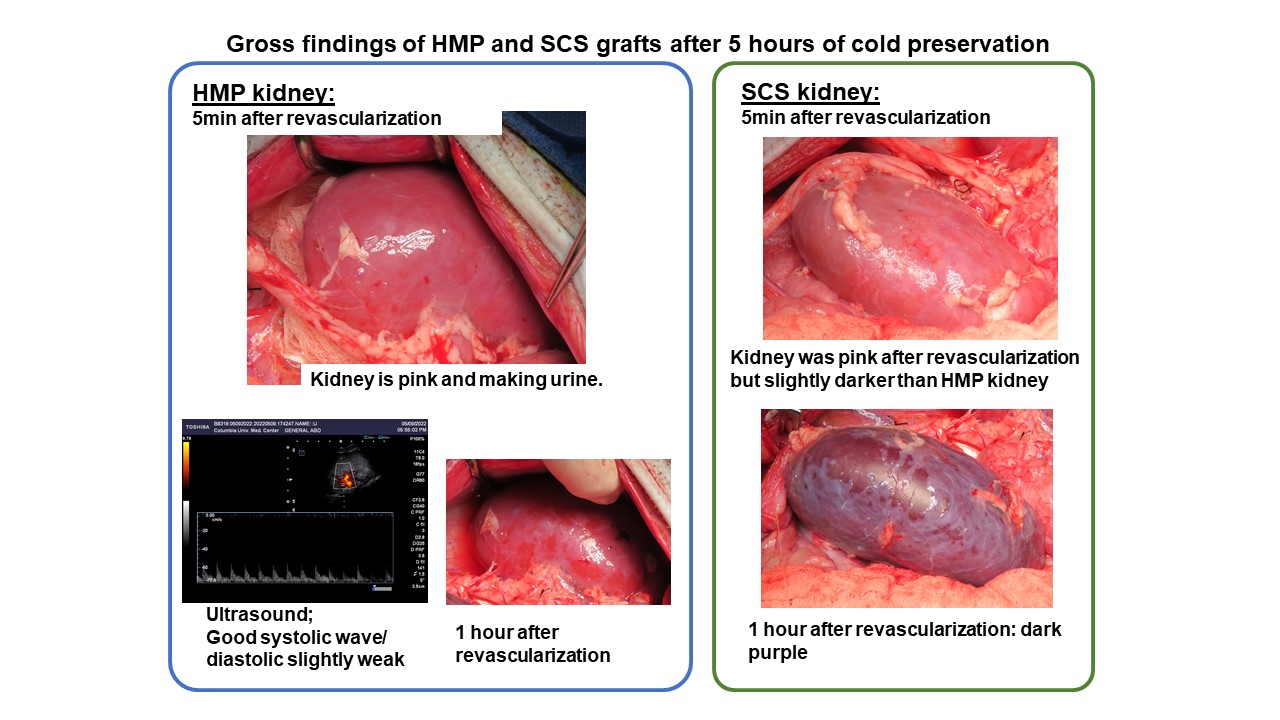
NIAID P01AI045897 Project1 Achieving Xenograft Tolerance through Thymic Programming in Primates.