Jonathan Fridell, United States
Chief, Abdominal Transplant Surgery
Surgery
Indiana University School of Medicine
Use of basiliximab as adjunct maintenance immunosuppressant in pancreas transplant recipients
Jeanne M Chen1, Asif A Sharfuddin2, John A Powelson3, Muhammad S Yaqub2, Andrew J Lutz3, Jonathan Fridell3.
1Pharmacy, IU Health, Indianapolis, IN, United States; 2Medicine, Indiana University School of Medicine, Indianapolis, IN, United States; 3Surgery, Indiana University School of Medicine, Indianapolis, IN, United States
Purpose: The objective of this review was to evaluate the safety of utilizing basiliximab in place of or in addition to standard adjunct immunosuppression medications (sirolimus &/or mycophenolic acid) following pancreas transplant.
Methods: This single center retrospective study reviewed the medical records of pancreas transplant recipients transplanted between January 1, 2007 and November 17, 2021 who received at least 3 months of basiliximab therapy with at least 1 year of follow-up.
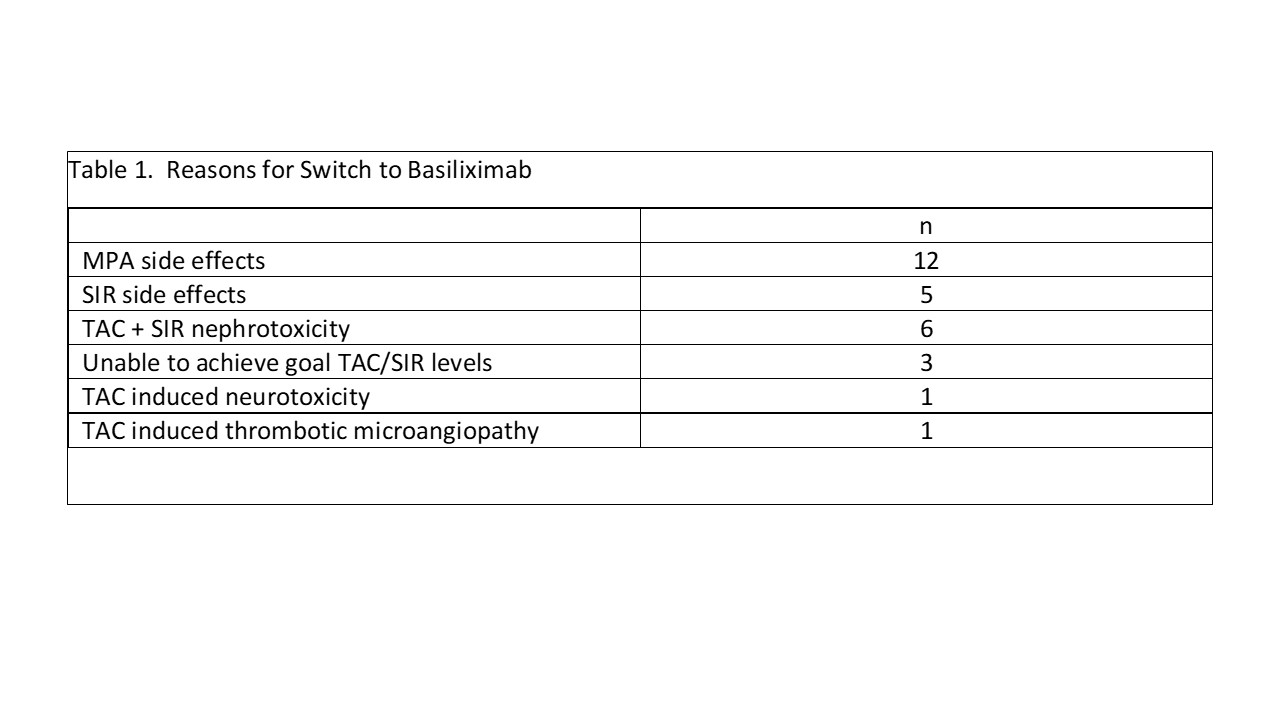
Results: 28 recipients; 5 kidney/pancreas (K/P) and 23 pancreas transplant alone (PTA) received anti-thymocyte globulin induction with tacrolimus (TAC) + sirolimus (SIR) for K/P or TAC + SIR + low dose mycophenolate (MPA) for PTA. A monthly IV infusion of basiliximab 40 mg was either added in addition to or replace adjunct immunosuppression or to spare TAC. Median time to switch was 14 mths (range 1-120 mths) following transplant. Primary reasons for switch are shown in table 1. Overall, there were 7 patients that had 9 episodes of pancreas rejection on therapy (2 requiring rATG), 7 pancreas graft losses, and 4 patient deaths – all occurred in PTA patients. Rejection occurred a median of 30 mths (range 4-100 mths) post conversion; graft loss a median of 8 (range 4-56) mths (table 2). Four patients died; two of 4 patients died with a functioning graft. All deaths occurred at least 1 year after discontinuation of basiliximab. Causes of death were intestinal hemorrhage (n=1), aspiration pneumonia (n=1), diabetic ketoacidosis (n=1), and unknown (n=1). No renal rejection or kidney graft losses were observed in K/P pts; 5 PTA developed ESRD (table 3). Two PTA patients developed CMV viremia while on basiliximab therapy; one also developed anal/vulvovaginal cancer. No patients developed BK virus infection. Fourteen patients remain on basiliximab therapy (4/5 K/P, 10/23 PTA) at a median of 29.5 mths (range 16-96 mths) post conversion with good pancreas and kidney function; 3 pts > 5 yrs.
Conclusion: Monthly basiliximab infusions can be considered as an alternative maintenance immunosuppressant agent in pancreas transplant recipients who have failed other conventional agents.
Lectures by Jonathan Fridell
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 14:00 - 15:30 |
Repeat pancreas transplant (IPITA) | Treatment Opinion 1: Pancreas Re-Transplantation / Senior | Indigo 204 |
|
Thu-26 09:00 - 12:00 |
Successfully navigating difficult medical and surgical scenarios in Pancreas Transplantation | Introduction to the session | Indigo D |
|
Thu-26 09:00 - 12:00 |
Successfully navigating difficult medical and surgical scenarios in Pancreas Transplantation | Immunologic allograft failure/pancreatectomy/retransplantation | Indigo D |
|
Thu-26 18:30 - 20:00 |
Poster Session | Use of Basiliximab as Adjunct Maintenance Immunosuppressant in Pancreas Transplant Recipients | Foyer Area |
