Columbia Center for Translational Immunology, Columbia University
Combined double thymus transplantation and mixed xenogeneic chimerism for the induction of optimal tolerance and enhancement of immune function
Soha Mcheik1, Tara Talaie1, Frederique van den Haak3, Hui Wang1, Hao Wei Li1, Xiaolan Ding1, Megan Sykes1,2,3.
1Department of Medicine, Columbia Center for Translational Immunology, New York, NY, United States; 2Department of Surgery, Columbia University, New York, NY, United States; 3Department of Microbiology and Immunology, Columbia University, New York, NY, United States
Introduction: Tolerance induction would avoid xenograft rejection and the need for toxic levels of non-specific immunosuppressive therapy. We have developed two complementary approaches to inducing human immune tolerance to the pig, namely mixed xenogeneic chimerism, which tolerizes T cells, B cells and NK cells, and porcine thymic transplantation, which induces T cell tolerance by combined clonal deletion and Treg function. However, in immunocompetent hosts, thymus transplantation requires recipient thymectomy. Furthermore, human T cells developing in porcine thymus grafts lack Tregs specific for human tissue-restricted antigens and have weak HLA-restricted antigen recognition. To circumvent these problems, we combined mixed xenogeneic chimerism with double thymus transplantation, modelling porcine thymic transplantation and mixed chimerism in a human without native thymectomy.
Methods: Thymectomized pig cytokine transgenic (PCT)-NSG mice received a thymocyte-depleted swine fetal thymus graft and a human fetal thymus graft under the capsule of each kidney. Human fetal liver CD34+ cells were given i.v. and half of the mice also received hCD47-transgenic pig bone marrow cells. Animals were sacrificed 19 weeks post-transplantation and thymocytes and splenocytes were analyzed and compared between groups. Splenic T cells, enriched by depletion of mouse cells and human B cells, were assessed for proliferative responses in CFSE-MLR.
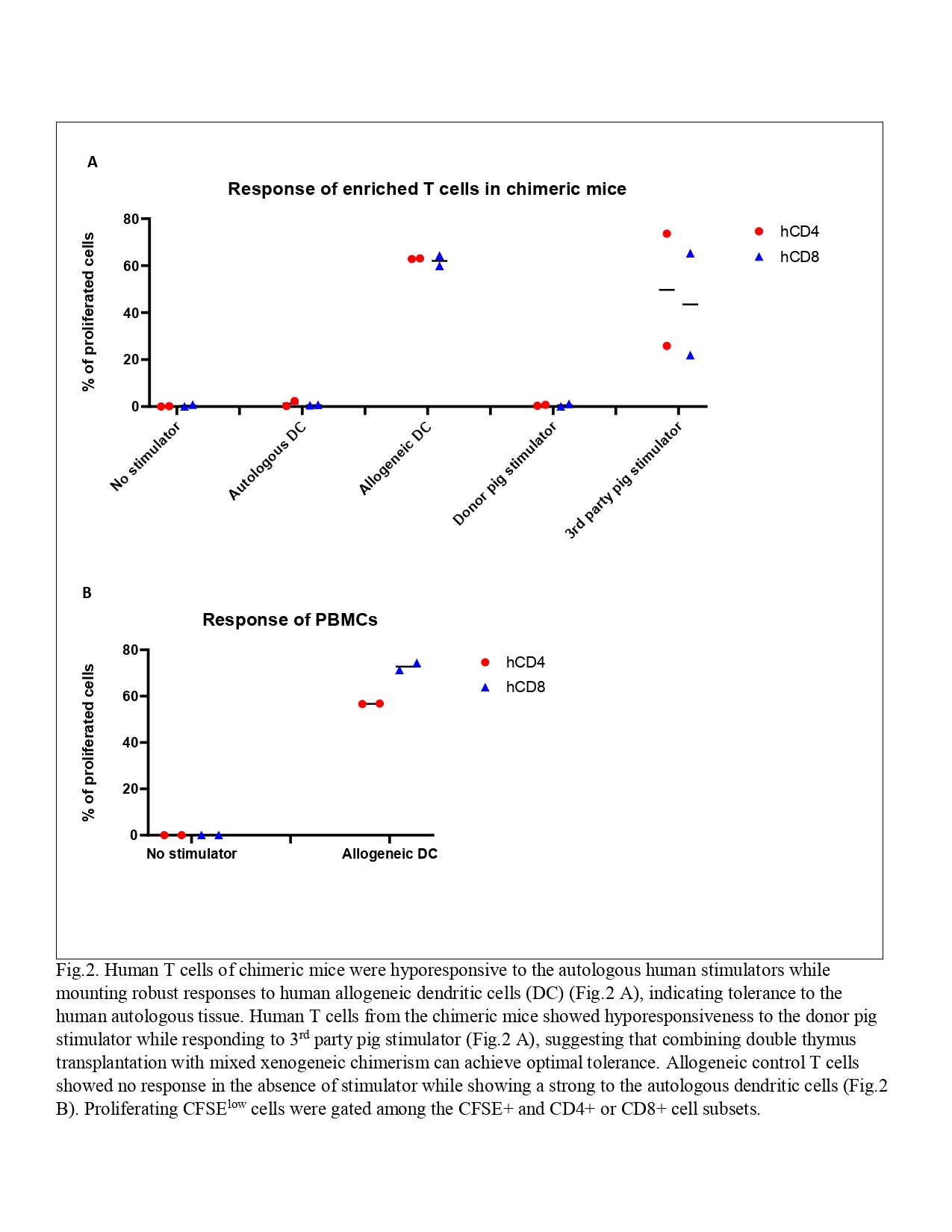
Results: Overall reconstitution of human cells (T cells, B cells and monocytes) in peripheral blood was delayed and reduced in mixed chimeric mice. Pig chimerism consisting mainly of T cells was persistent in chimeric mice. Overall human cell reconstitution was also decreased in spleen and bone marrow in chimeric compared to non-chimeric mice. Pig chimerism was detected in both spleen and bone marrow of chimeric mice. Human T cells from both groups were hyporesponsive to autologous human stimulators while mounting robust responses to human allogeneic stimulators (Fig.1 and Fig.2 A), indicating tolerance to the human autologous antigens. Allogeneic control T cells showed a strong response to the autologous dendritic cells (Fig.2 B). While human T cells from chimeric mice showed specific hyporesponsiveness to the donor pig with robust third-party pig responses (Fig.2 A), human T cells from two of three non-chimeric mice proliferated robustly to the donor pig (Fig.1). These data suggest that specific human T cell tolerance to the donor pig xenoantigens was achieved in the presence of mixed chimerism and a human plus a porcine thymus.
Conclusion: Our data suggest that combining porcine thymus transplantation with mixed xenogeneic chimerism may achieve optimal tolerance while ensuring recognition of pathogens in the context of human and porcine MHC molecules without requiring thymectomy of the host.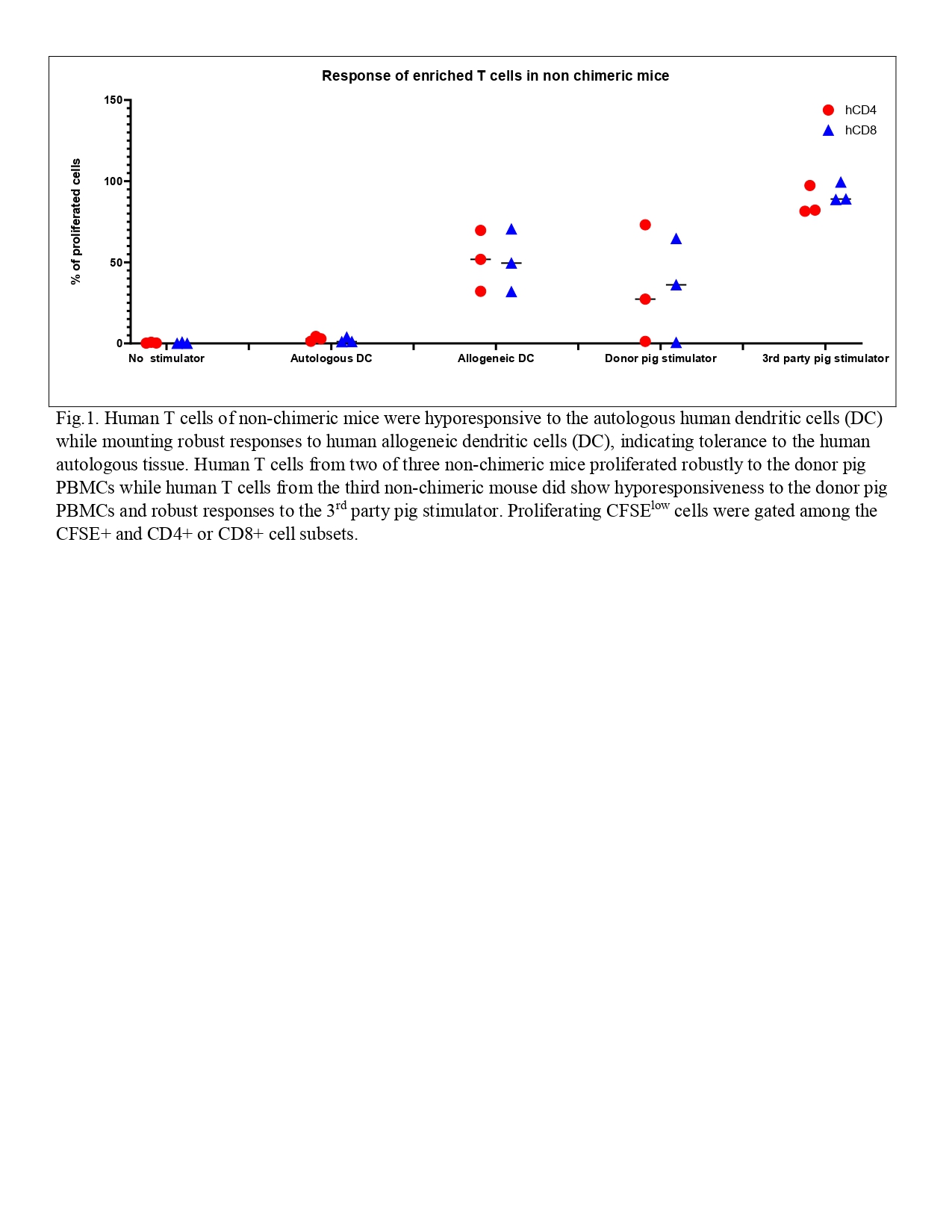
We thank David H. Sachs and Harko Mulder for providing pig tissues.. This work was supported by Xeno/PPG grant P01AI045897.. Research reported in this abstract was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10RR027050 and S10OD020056. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health..
Lectures by Soha Mcheik
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 10:00 - 11:30 |
Immune monitoring for rejection / Immunomodulation / tolerance | Combined double thymus transplantation and mixed xenogeneic chimerism for the induction of optimal tolerance and enhancement of immune function | Indigo 204 |
