Cliniques Universitaires Saint Luc
Continuous vs Discontinuous Purification of Isolated Human Islets: Functional and Morphological Comparison
Antoine Buemi1,2, Nizar Mourad2, Tom Darius1,2, Arnaud Devresse1, Nada Kanaan1, Pierre Gianello2, Michel Mourad1,2.
1Transplant Surgery, Cliniques Universitaires Saint Luc, Bruxelles, Belgium; 2Pole de Chirurgie Experimentale, Université Catholique de Louvain, Bruxelles, Belgium
Background: The COBE 2991 cell processor, commonly used for pancreatic islet isolation, is no longer distributed in Europe, leading to search for alternative purification procedures with equivalent efficacy.
The aim of this study was to evaluate the efficacy of an alternative method based on the discontinuous purification of islets.
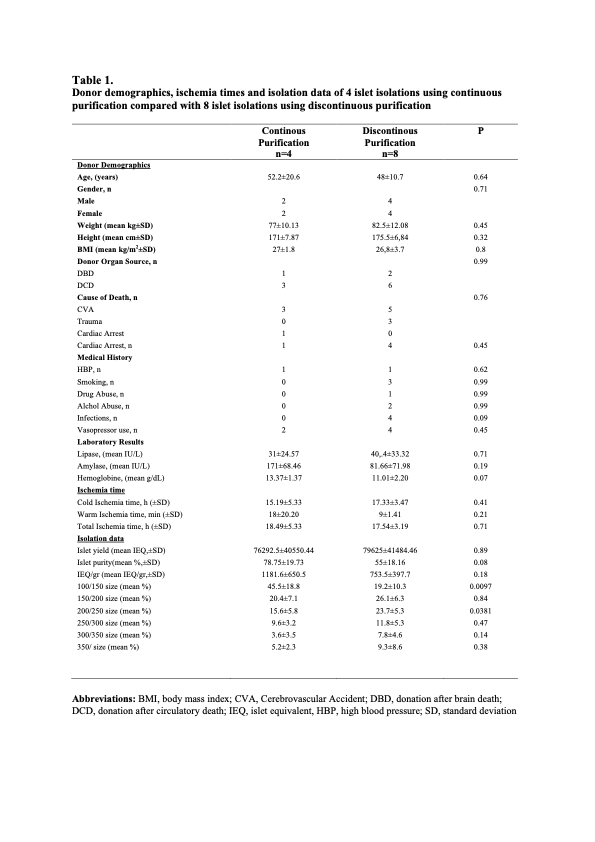
Methods: The conventional isolation procedure using a standard continuous islet purification with COBE 2991 of n=4 human pancreas was compared to n=8 procedures using a discontinuous purification with a “bottle” method from donors of similar characteristics.
Islet equivalents, purity and dynamic glucose-stimulated insulin secretion were evaluated.
Results: A similar islet yield was obtained using continuous vs discontinuous purification methods (76292.5±40550.44 versus 79625±41484.46 islet equivalents, P =0.89). Islets from both groups had similar purity (78.75%±19.73% vs 55%±18.16%, P=0.08) and functionality both in terms of stimulation index (3.31±0.83 vs 5.58±3.38, P=0.22) and insulin secretion (1.26±0.83 vs 1.53±1.40 mean AUC, P=0.73).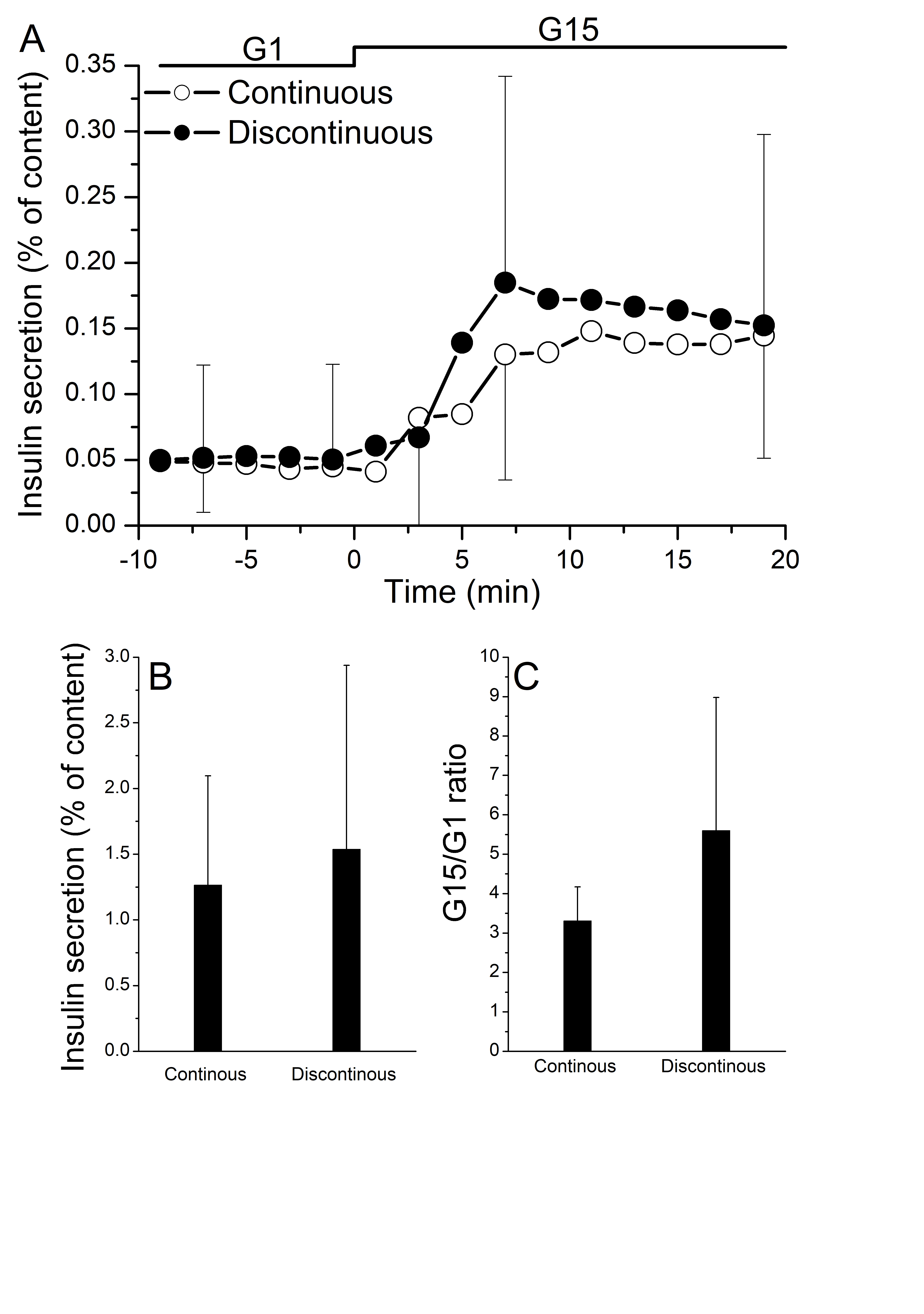
Moreover, the size of the islets was significantly larger in the discontinuous vs continuous purification group (19.2%±10.3% vs 45.4%±18.8% of islets less then 100mm, P=0.0097 and 23.7%±5.3% vs 15.6%±5.8 % of 200-250mm islets size, P=0.03).
Conclusions: Compared to the conventional purification procedure, discontinuous purification with a “bottle” method shows similar results with regards to isolation yield and islet secretory function. Furthermore, this alternative technique allows obtaining larger islets.
References:
[1] 1. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–238.
2. Ricordi C, Lacy PE, Finke EH, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420.
3. Kin T. Islet isolation for clinical transplantation. Adv Exp Med Biol. 2010;654:683–710.
4. Lake SP, Bassett PD, Larkins A, et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes. 1989;38:143–145.
5. Scharp, D.W.; Lacy, P.E.; Finke, E.; Olack, B. Low-temperature culture of human islets isolated by the distention method and purified with Ficoll or Percoll gradients. Surgery 1987, 102, 869–879.
6. Kin T. Islet isolation for clinical transplantation. Adv Exp Med Biol 2010; 654:683-710; PMID:20217520; http://dx.doi.org/10.1007/978-90-481-3271-3_30
7. Mourad NI, Perota A, Xhema D, Galli C, Gianello P. Transgenic Expression of Glucagon-Like Peptide-1 (GLP-1) and Activated Muscarinic Receptor (M3R) Significantly Improves Pig Islet Secretory Function. Cell Transplant. 2017 May 9;26(5):901-911. doi: 10.3727/096368916X693798. Epub 2016 Nov 22. PMID: 27938490; PMCID: PMC5657716.
8. Wang LJ, Kin T, O'Gorman D, Shapiro AMJ, Naziruddin B, Takita M, Levy MF, Posselt AM, Szot GL, Savari O, Barbaro B, McGarrigle J, Yeh CC, Oberholzer J, Lei J, Chen T, Lian M, Markmann JF, Alvarez A, Linetsky E, Ricordi C, Balamurugan AN, Loganathan G, Wilhelm JJ, Hering BJ, Bottino R, Trucco M, Liu C, Min Z, Li Y, Naji A, Fernandez LA, Ziemelis M, Danobeitia JS, Millis JM, Witkowski P. A Multicenter Study: North American Islet Donor Score in Donor Pancreas Selection for Human Islet Isolation for Transplantation. Cell Transplant. 2016;25(8):1515-1523. doi: 10.3727/096368916X691141. Epub 2016 Feb 26. PMID: 26922947; PMCID: PMC5167495.
9. Henquin JC, Dufrane D, Kerr-Conte J, Nenquin M. Dynamics of glucose-induced insulin secretion in normal human islets. Am J Physiol Endocrinol Metab. 2015 Oct 1;309(7):E640-50. doi: 10.1152/ajpendo.00251.2015. Epub 2015 Aug 11. PMID: 26264556.
10. Noguchi H. Pancreatic Islet Purification from Large Mammals and Humans Using a COBE 2991 Cell Processor versus Large Plastic Bottles. J Clin Med. 2020 Dec 23;10(1):10. doi: 10.3390/jcm10010010. PMID: 33374512; PMCID: PMC7793136.
Lectures by Antoine Buemi MD
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 11:35 - 12:35 |
Miscellaneous topics 2 | Continuous vs Discontinuous Purification of Isolated Human Islets: Functional and Morphological Comparison | Indigo D |
|
Sat-28 16:00 - 17:30 |
Organ and tissue preservation, cryopreservation and reconditioning | Exploring preservation modalities in a split human pancreas model to investigate the effect on the islet isolation outcomes. | Indigo H |
