Islet encapsulation preserves their functionality following transplantation into immunocompetent diabetic animals without immunosuppression
Harald Stover1.
1Allarta Life Science Inc., Hamilton, ON, Canada
Background and aims: The loss of insulin-producing beta cells in the pancreas characterizes type 1 diabetes (T1D). Despite the promise of islet cell transplantation to replace lost cells and cure T1D, the negative effects of systemic immune suppression remain a significant issue, and long-term tight glycemic control remains difficult to achieve. We developed proprietary synthetic hydrogels that are resilient, shape agnostic, and non-immunogenic to enable allogenic islet cell transplantation.
Materials and methods: The mechanical robustness and other properties of hydrogel beads, strings and larger 3D structures were examined by micropipette aspiration, chemical stress tests (e.g. citrate), and in vivo persistence. FITC-labeled dextran (10, 70, 250, and 500 kDa), IgG (150 kDa), and insulin (5.8 kDa) were used to assess protein release profiles and diffusion limits. Human induced pluripotent stem cells (hiPSCs) were assessed for viability and metabolic activity in various hydrogel formulations, and healthy rodents and pigs were used to test for fibrosis. After encapsulation, islet function was evaluated through in vitro glucose-stimulated insulin secretion (GSIS). Following implantation into streptozotocin (STZ)-induced diabetic immunocompetent mice and healthy normal mixed breed pigs, hydrogels with marginal islet loads were examined for fibrosis, blood glucose control, C-peptide, and glycated hemoglobin (HbA1c). For allogenic transplants, encapsulated rat islets were implanted into healthy rats.
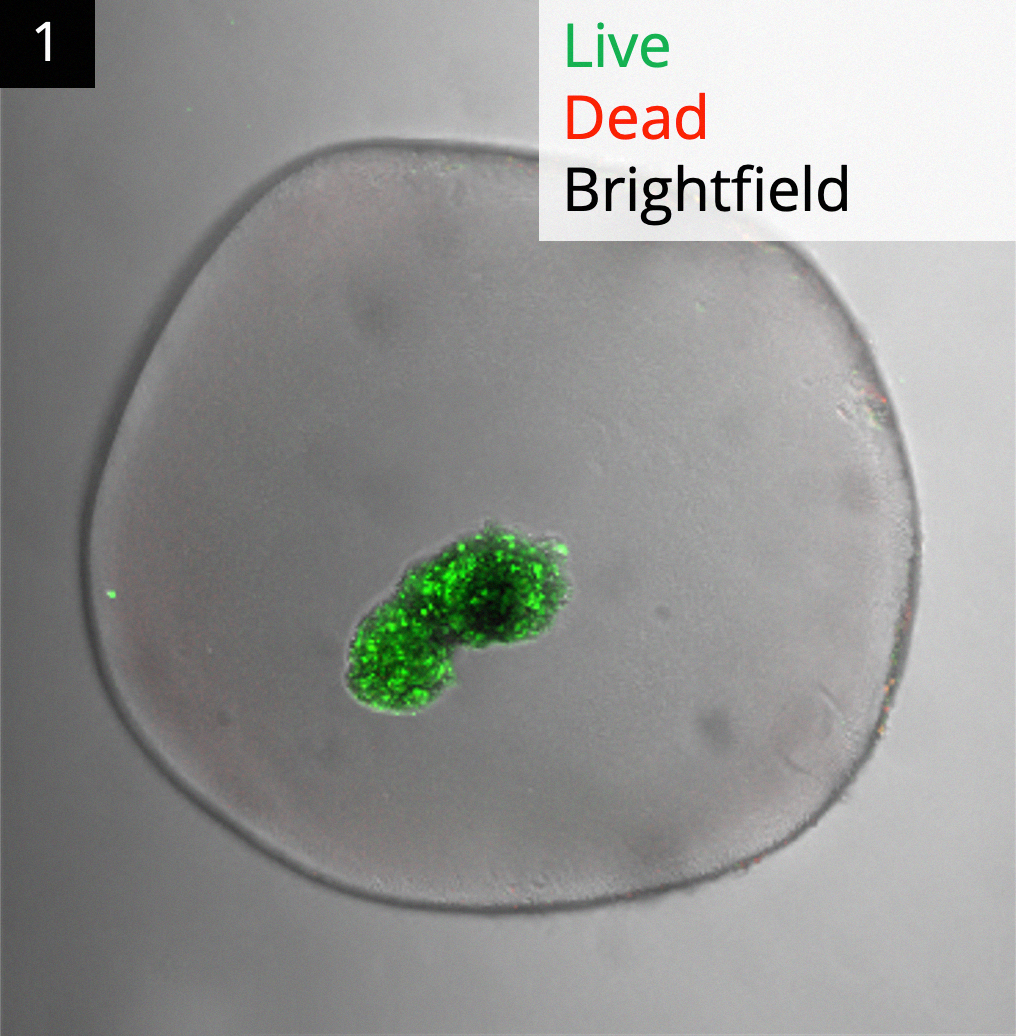
Results: The hydrogels are resilient and can easily form beads, strings, and larger 3D structures. They exclude IgGs and larger molecules while allowing rapid insulin out-diffusion (t1/2 = 1 min). Several immune-evasive modifications increased the cell viability of encapsulated hiPSCs and islets, and reduced immunogenicity in vivo. Empty hydrogel beads and strings were durable and retrievable following implantation, showing only minimal foreign body response. High viability and GSIS indices from rat and donor human islets show that the hydrogels with different form factors preserved islet function in vitro. Using a marginal xenogenic islet loads, encapsulated rat islets enabled rapid and sustained blood glucose control (250 mg/dL) in immunocompetent STZ-diabetic C57BL/6 mice. Viable human donor islets produced C-peptide readings (up to 45 pmol/L) in such mice for 45 days (~8x longer than free human islets). Fig. 1 shows viable human islets explanted from healthy pigs. Explanted hydrogels with allogeneic islets showed no fibrosis in healthy rats, demonstrating the potential of this technology for therapeutic cell transplantations.
Conclusion: Our proprietary synthetic hydrogels protected xenogenic islet transplants in immune-competent animals, including pigs. While xenografts showed some fibrosis, especially for failing islets, allografts showed limited fibrosis, if any. We are currently scaling to therapeutic doses in large animals using easily retrievable shape factors in search of a curative human allogeneic therapy for T1D that does not require immunosuppression.
Lectures by Harald Stover
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 14:00 - 15:30 |
Encapsulation of cellular transplants 1 | Islet encapsulation preserves their functionality following transplantation into immunocompetent diabetic animals without immunosuppression. | Indigo D |
