A novel endovascular bioartificial pancreas device to treat type 1 diabetes
Sara Photiadis1, Khanh Hoa Nguyen1, Quynh Mai1, Gabriel Montanez1.
1Isla Technologies, Inc., San Carlos, CA, United States
Previous research and rationale Type 1 diabetes (T1D) (1, 2) is an autoimmune disease that causes destruction of insulin-producing beta-cells within the islets of Langerhans in the pancreas. Bioartificial pancreas (BAP) devices with immune-protecting barriers for islet transplantation are an emerging T1D treatment strategy (3-7), but these devices remain challenging to implement due to (i) poor islet graft survival in low oxygen subcutaneous environments and (ii) suboptimal transport glucose and insulin for metabolic control (8-15).
Isla Technologies’ (Isla) T1D treatment strategy is an endovascular BAP (eBAP) device, which overcomes existing challenges in immunoisolation (16, 17) and hypoxia-induced islet death (18) through a novel approach of delivering islets within immune-protective membranes on a stent scaffold to a peripheral artery. The eBAP device leverages the artery’s (i) higher oxygen environment and (ii) blood flow to improve diffusion of glucose and insulin through the device (19). To demonstrate feasibility, we implanted the eBAP into a non-diabetic swine model without immunosuppression.
Methods Non-diabetic, 70 Kg Yorkshire swine (N=5) were implanted with eBAP devices without immunosuppression. Group 1 received the eBAP device with sterile alginate alone (N=2). Group 2 received the eBAP device with sterile alginate and 50,000 juvenile porcine islet equivalents (N=3). The devices were implanted using a 20 Fr catheter through the femoral artery to the infrarenal aorta. Angiograms were obtained over 1 month to evaluate the formation of blood clots and explanted devices were histologically examined for islet endocrine markers and viability.
Results We achieved 100% patency in all control devices and devices with islets. In Figure 1, the angiograms on panel A represent day 0 and on panel B, day 30 for an implant with islets. Explanted islet-containing devices at day 30 were evaluated for gross and microscopic histopathology. In Figure 1C, the blood contacting surface of the device is shown, indicating minimal inflammatory response. The histological sections show positive staining with antibodies against human insulin and glucagon and lack of caspase 3 staining in islets. (Figure 1D).
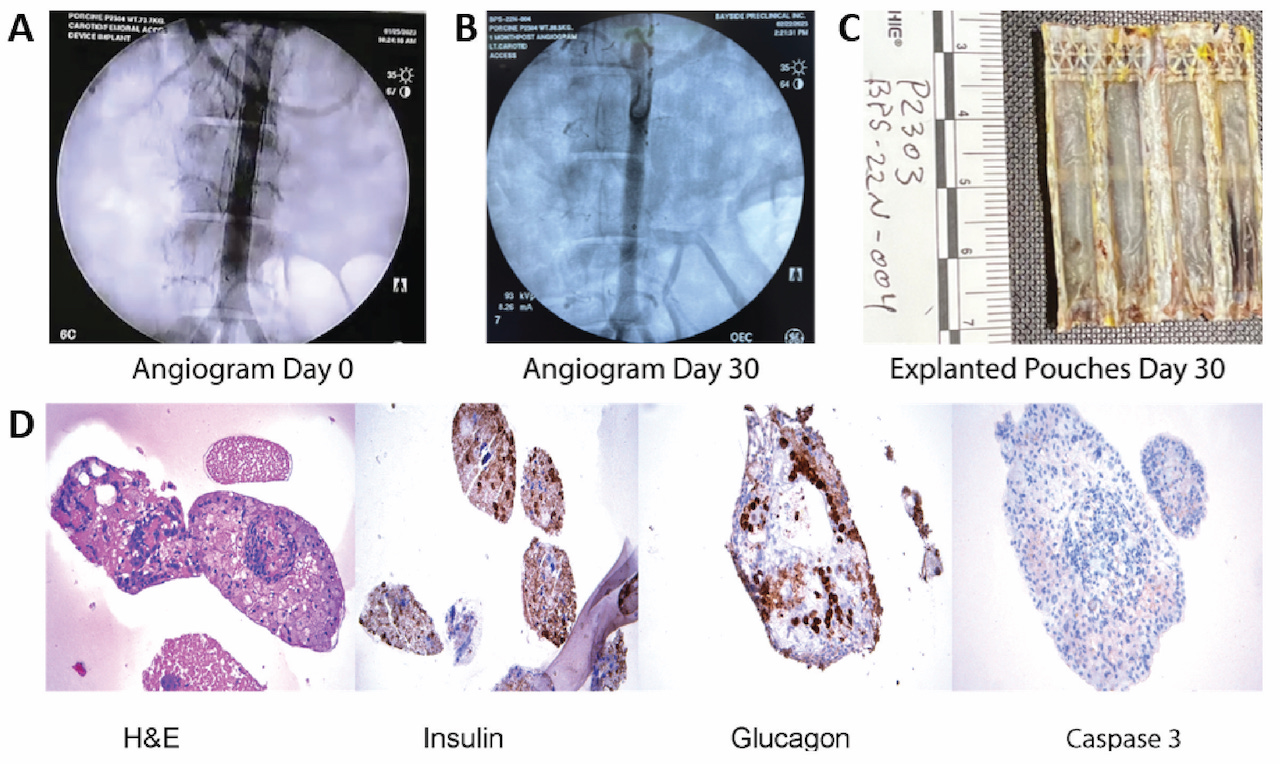
Conclusion We show 30-day safety data of a novel eBAP device delivered endovascularly to a peripheral artery in non-diabetic swine without immunosuppression. The eBAP device with and without allogeneic islets showed no evidence of thrombosis over 30 days and histopathology indicated endocrine functionality and viability of the islets. Further investigation is warranted to test the ability of the eBAP device to reverse diabetes in vivo and to assess its extended safety profile.
Thomas Krebs. Alice Tomei. Jonathan Lakey.
References:
[1] American Diabetes Association. Statistics About Diabetes. Statistics. 2022.
[2] Centers for Disease Control and Prevention. Prevalence of Diagnosed Diabetes. National Diabetes Statistics Report. 2022.
[3] Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci U S A. 2013;110(47):19054-8.
[4] Safley SA, Cui H, Cauffiel S, Tucker-Burden C, Weber CJ. Biocompatibility and immune acceptance of adult porcine islets transplanted intraperitoneally in diabetic NOD mice in calcium alginate poly-L-lysine microcapsules versus barium alginate microcapsules without poly-L-lysine. J Diabetes Sci Technol. 2008;2(5):760-7.
[5] Schweicher J, Nyitray C, Desai TA. Membranes to achieve immunoprotection of transplanted islets. Front Biosci (Landmark Ed). 2014;19(1):49-76.
[6] Syed F, Bugliani M, Novelli M, Olimpico F, Suleiman M, Marselli L, et al. Conformal coating by multilayer nano-encapsulation for the protection of human pancreatic islets: In-vitro and in-vivo studies. Nanomedicine. 2018;14(7):2191-203.
[7] Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, et al. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl Med. 2015;4(10):1214-22.
[8] Song S, Blaha C, Moses W, Park J, Wright N, Groszek J, et al. An intravascular bioartificial pancreas device (iBAP) with silicon nanopore membranes (SNM) for islet encapsulation under convective mass transport. Lab Chip. 2017;17(10):1778-92.
[9] Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci U S A. 2012;109(13):5022-7.
[10] Barkai U, Weir GC, Colton CK, Ludwig B, Bornstein SR, Brendel MD, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2013;22(8):1463-76.
[11] Pedraza E, Coronel M, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. . Proc Natl Acad Sci. 2012;19:4245–50.
[12] Evron Y, Zimermann B, Ludwig B, Barkai U, Colton CK, Weir GC, et al. Oxygen supply by photosynthesis to an implantable islet cell device. Horm Metab Res. 2015;47(1):24-30.
[13] Avgoustiniatos ES, Colton CK. Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue. Ann N Y Acad Sci. 1997;831:145-67.
[14] de Groot M, Schuurs TA, Keizer PP, Fekken S, Leuvenink HG, van Schilfgaarde R. Response of encapsulated rat pancreatic islets to hypoxia. Cell Transplant. 2003;12(8):867-75.
[15] Colton CK. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev. 2014;67-68:93-110.
[16] De Toni T, Stock AA, Devaux F, Gonzalez GC, Nunez K, Rubanich JC, et al. Parallel Evaluation of Polyethylene Glycol Conformal Coating and Alginate Microencapsulation as Immunoisolation Strategies for Pancreatic Islet Transplantation. Frontiers in Bioengineering and Biotechnology. 2022;10.
[17] Desai TA, Chu WH, Tu JK, Beattie GM, Hayek A, Ferrari M. Microfabricated immunoisolating biocapsules. Biotechnol Bioeng. 1998;57(1):118-20.
[18] Chen J, Chen J, Cheng Y, Fu Y, Zhao H, Tang M, et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Research & Therapy. 2020;11(1):97.
[19] Song S, Faleo G, Yeung R, Kant R, Posselt AM, Desai TA, et al. Silicon nanopore membrane (SNM) for islet encapsulation and immunoisolation under convective transport. Scientific Reports. 2016;6(1):23679.
Lectures by Sara Photiadis
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 14:00 - 15:30 |
Encapsulation of cellular transplants 1 | A novel endovascular bioartificial pancreas device to treat type 1 diabetes | Indigo D |
