Mohamed A Elzawahry, United Kingdom
Doctoral student
Nuffield Department of surgical sciences
University of Oxford
Oxygenated hypothermic machine perfusion of the pancreas; a porcine circulatory death model to compare a ‘continuous’ and an ‘end-ischaemic’ approach
Mohamed A Elzawahry1,2, John Fallon1,2, Sameena Nawaz3, Benoit Hastoy3, Anne Clark3, Peter J Friend1,2, Rutger J Ploeg1,2, James Hunter1,2.
1Nuffield Department of Surgical sciences, University of Oxford, Oxford, United Kingdom; 2Oxford Transplant Center, Churchill Hospital, Oxford University Hospitals, Oxford, United Kingdom; 3Oxford Centre for Diabetes, Endocrinology and Metabolism, University of Oxford, Oxford, United Kingdom
Introduction: Beta cell replacement is an ongoing challenge. Solid organ pancreas and islet cell transplantation are used in the treatment of a very selective group of patients with diabetes (1). Pancreases are extremely vulnerable to ischaemia-reperfusion injury (IRI) (2). During static cold storage (SCS) ATP is depleted, and by-products of anaerobic respiration, including succinate and lactate, accumulate. This injury affects quantity and viability of isolated islets and in whole-organ transplantation, it is characterised by acinar necrosis, oedema, and endothelial disruption (3–5) (graft pancreatitis) after implantation. Recently, the introduction of oxygenated hypothermic machine perfusion (HMPO2) in clinical liver and kidney preservation has demonstrated a significant reduction in the consequences of IRI (6,7). Pancreas HMPO2 was shown to be feasible and potentially beneficial in several pre-clinical studies (8–14). The aim of this study is to compare a ‘continuous’ to an ‘end-ischaemic’ approach in application of HMPO2 in pancreases using a porcine circulatory death model.
Methods: Porcine pancreases were retrieved from the abattoir after exsanguination. Pancreases were flushed with UW cold storage solution and prepared surgically, then either started on HMPO2 for the totality of the cold storage time (‘continuous’ group, n=6) or preserved in SCS then on HMPO2 for the last two hours of the cold storage time (‘end’ group, n=6), using oxygenated UW machine perfusion solution. Both groups then underwent normothermic reperfusion (NR) with autologous whole blood to mimic transplantation and instigate IRI. Using modified Xvivo Organ Assist perfusion systems. Perfusate samples were taken at regular intervals throughout the experiment and glucose stimulated insulin secretion (GSIS) was measured during NR using Mercodia human insulin ELISA (n=3 in each group).
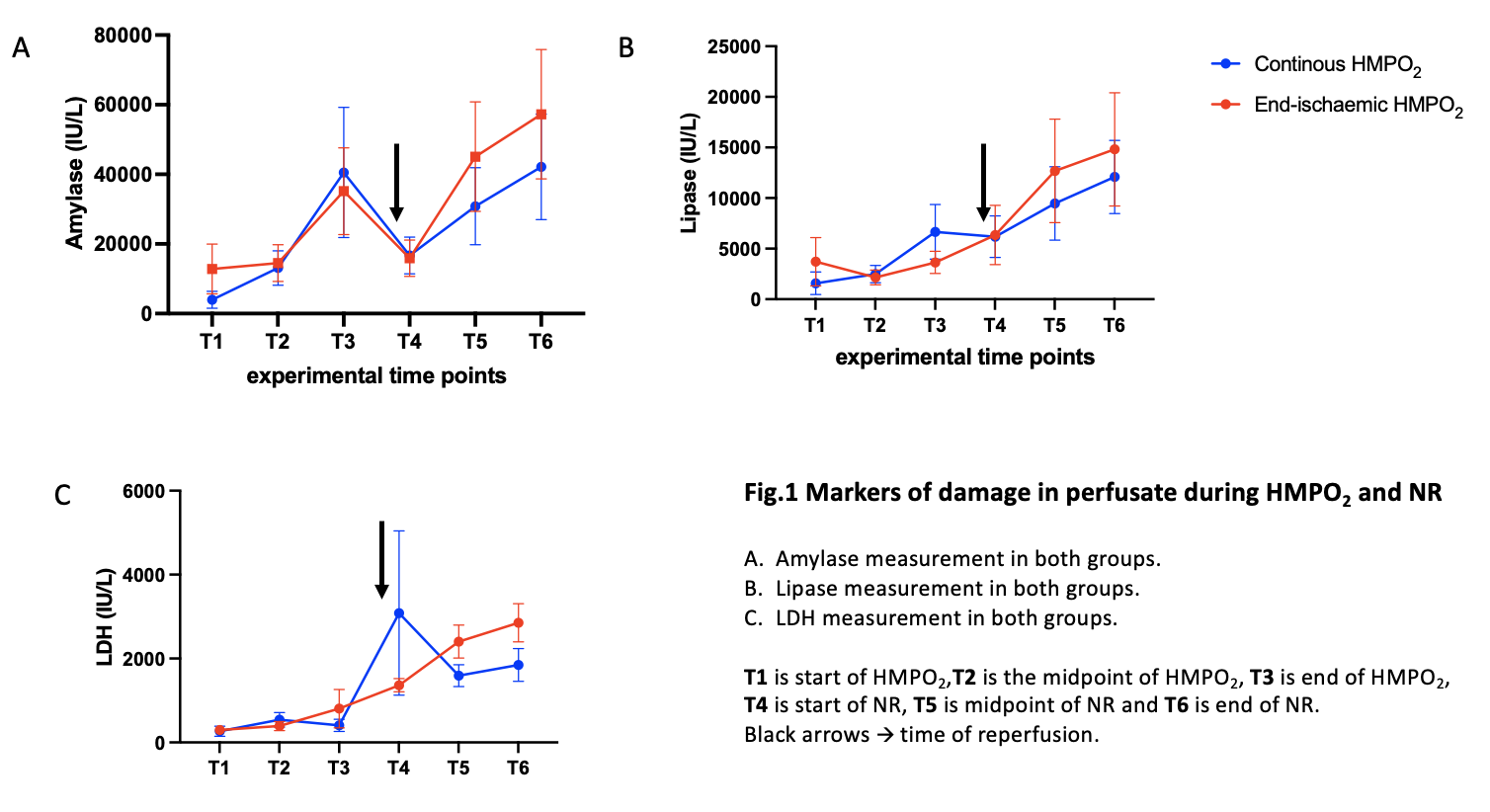
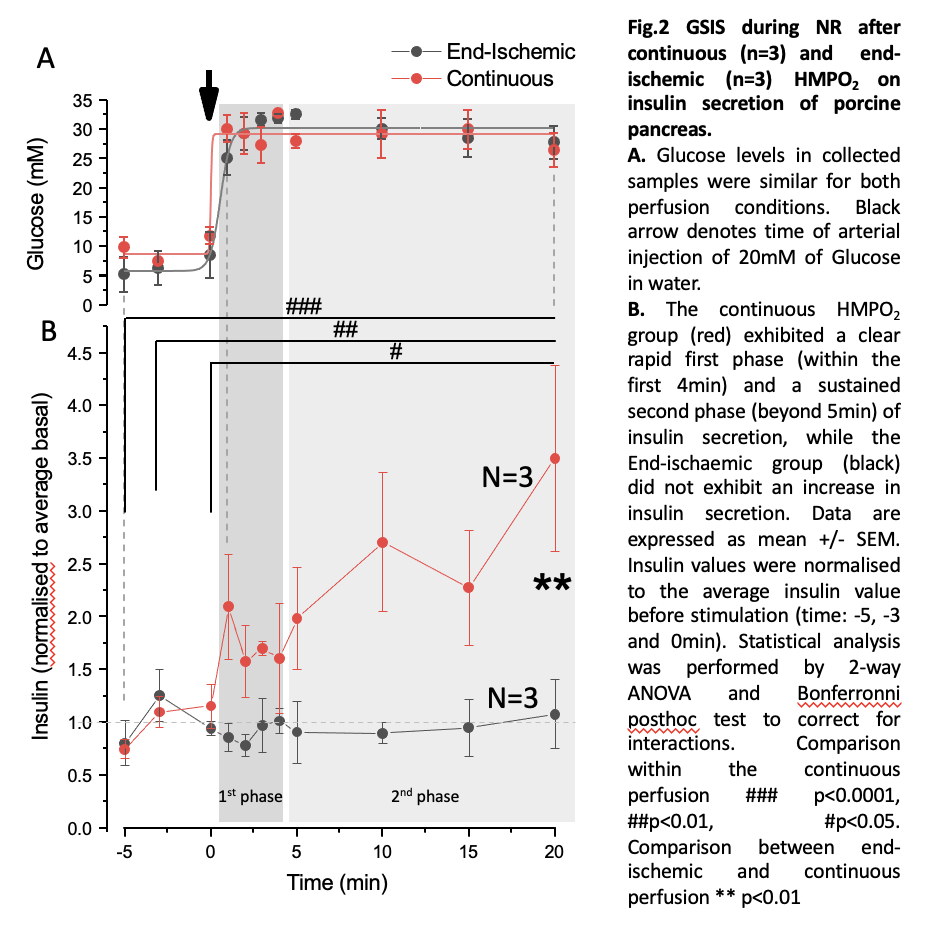
Results: The continuous group had a mean warm ischaemia time (WIT) of 18 minutes and a mean cold ischaemia time (CIT) of 8 hours 11 minutes, while the end group had a mean WIT of 19 minutes and mean CIT of 8 hours 48 minutes. Both groups had no significant change of wet-to-dry ratio during the experiment, despite an increase in gross weight. Flows were higher throughout both HMPO2 and NR in the continuous group. Amylase, Lipase and LDH increased throughout the study for all pancreases with a peak at the end of the CIT (fig.1) and showed no statistically significant difference between both groups. The continuous group had a significantly greater insulin secretion in response to glucose stimulation and followed a biphasic pattern (fig.2).
Conclusion: Continuous HMPO2 preserved islet function and was a superior mode of preservation in a circulatory death porcine pancreas model, showing no statistically significant difference in parenchymal oedema or perfusate markers of tissue damage, along with improved perfusion parameters.
Funding for this project was provided by the 'Oxford Transplant Foundation'..
References:
[1] Stratta RJ, Gray D, Friend P, Colman A. Pancreas, islet and stem cell transplantation for diabetes. Oxford university press; 2010.
[2] Dholakia S. Assessment and optimisation of the pancreas allograft [PhD Thesis]. University of Oxford; 2019.
[3] Farney AC, Rogers J, Stratta RJ. Pancreas graft thrombosis: causes, prevention, diagnosis, and intervention. Current Opinion in Organ Transplantation. 2012 Feb;17(1):87–92.
[4] Muthusamy ASR, Mumford L, Hudson A, Fuggle SV, Friend PJ. Pancreas Transplantation From Donors After Circulatory Death From the United Kingdom: DCD Pancreas Transplantation in the UK. American Journal of Transplantation. 2012 Aug;12(8):2150–6.
[5] Troppmann C. Complications after pancreas transplantation: Current Opinion in Organ Transplantation. 2010 Feb;15(1):112–8.
[6] van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, et al. Hypothermic Machine Perfusion in Liver Transplantation — A Randomized Trial. N Engl J Med. 2021 Apr 15;384(15):1391–401.
[7] Jochmans I, Brat A, Davies L, Hofker HS, van de Leemkolk FEM, Leuvenink HGD, et al. Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): a randomised, double-blind, paired, phase 3 trial. The Lancet. 2020 Nov;396(10263):1653–62.
[8] Branchereau J, Renaudin K, Kervella D, Bernadet S, Karam G, Blancho G, et al. Hypothermic pulsatile perfusion of human pancreas: Preliminary technical feasibility study based on histology. Cryobiology. 2018 Dec;85:56–62.
[9] Leemkuil M, Lier G, Engelse MA, Ploeg RJ, de Koning EJP, ‘t Hart NA, et al. Hypothermic Oxygenated Machine Perfusion of the Human Donor Pancreas. Transplantation Direct. 2018 Oct;4(10):e388.
[10] Hamaoui K, Gowers S, Sandhu B, Vallant N, Cook T, Boutelle M, et al. Development of pancreatic machine perfusion: translational steps from porcine to human models. Journal of Surgical Research. 2018 Mar;223:263–74.
[11] Prudhomme T, Renaudin K, Lo Faro ML, Cantarovich D, Kervella D, Minault D, et al. Ex situ hypothermic perfusion of nonhuman primate pancreas: A feasibility study. Artif Organs. 2020 Jul;44(7):736–43.
[12] Prudhomme T, Kervella D, Ogbemudia AE, Gauttier V, Le Bas‐Bernardet S, Minault D, et al. Successful pancreas allotransplantations after hypothermic machine perfusion in a novel diabetic porcine model: a controlled study. Transpl Int. 2021 Feb;34(2):353–64.
[13] Doppenberg JB, Leemkuil M, Engelse MA, Krikke C, Koning EJP, Leuvenink HGD. Hypothermic oxygenated machine perfusion of the human pancreas for clinical islet isolation: a prospective feasibility study. Transpl Int. 2021 Aug;34(8):1397–407.
[14] Ogbemudia AE, Hakim G, Dengu F, El‐Gilani F, Dumbill R, Mulvey J, et al. Development of ex situ normothermic reperfusion as an innovative method to assess pancreases after preservation. Transpl Int. 2021 Sep;34(9):1630–42.
Lectures by Mohamed A Elzawahry
| When | Session | Talk Title | Room |
|---|---|---|---|
|
Sat-28 16:00 - 17:30 |
Organ and tissue preservation, cryopreservation and reconditioning | Oxygenated hypothermic machine perfusion of the pancreas; a porcine circulatory death model to compare a ‘continuous’ and an ‘end-ischaemic’ approach | Indigo H |
