Development of a novel method for measuring tissue oxygen pressure to improve the hypoxic condition in subcutaneous islet transplantation
Hiroaki Mitsugashira1, Takehiro Imura2, Akiko Inagaki2, Shoki Suzuki1, Yukiko Endo1, Takumi Katano2, Ryusuke Saito1, Kazuaki Tokodai1, Kimiko Watanabe2, Takashi Kamei1, Michiaki Unno1, Masafumi Goto1,2.
1Department of Surgery, Tohoku University Graduate School of Medicine, Sendai, Japan; 2Division of Transplantation and Regenerative Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan
Introduction: Subcutaneous tissue is a promising site for islet transplantation, but poor engraftment, due to hypoxia and low vascularity, hinders its prevalence. However, oxygen partial pressure (pO2) of the subcutaneous space (SC) and other sites like the renal subcapsular space (RS) were reported to be equivalent in several previous reports. This contradiction may be based on accidental puncture to the indwelling micro-vessels in target tissues. We therefore developed a novel optical sensor system for measuring tissue pO2 and estimated the utility of this method.
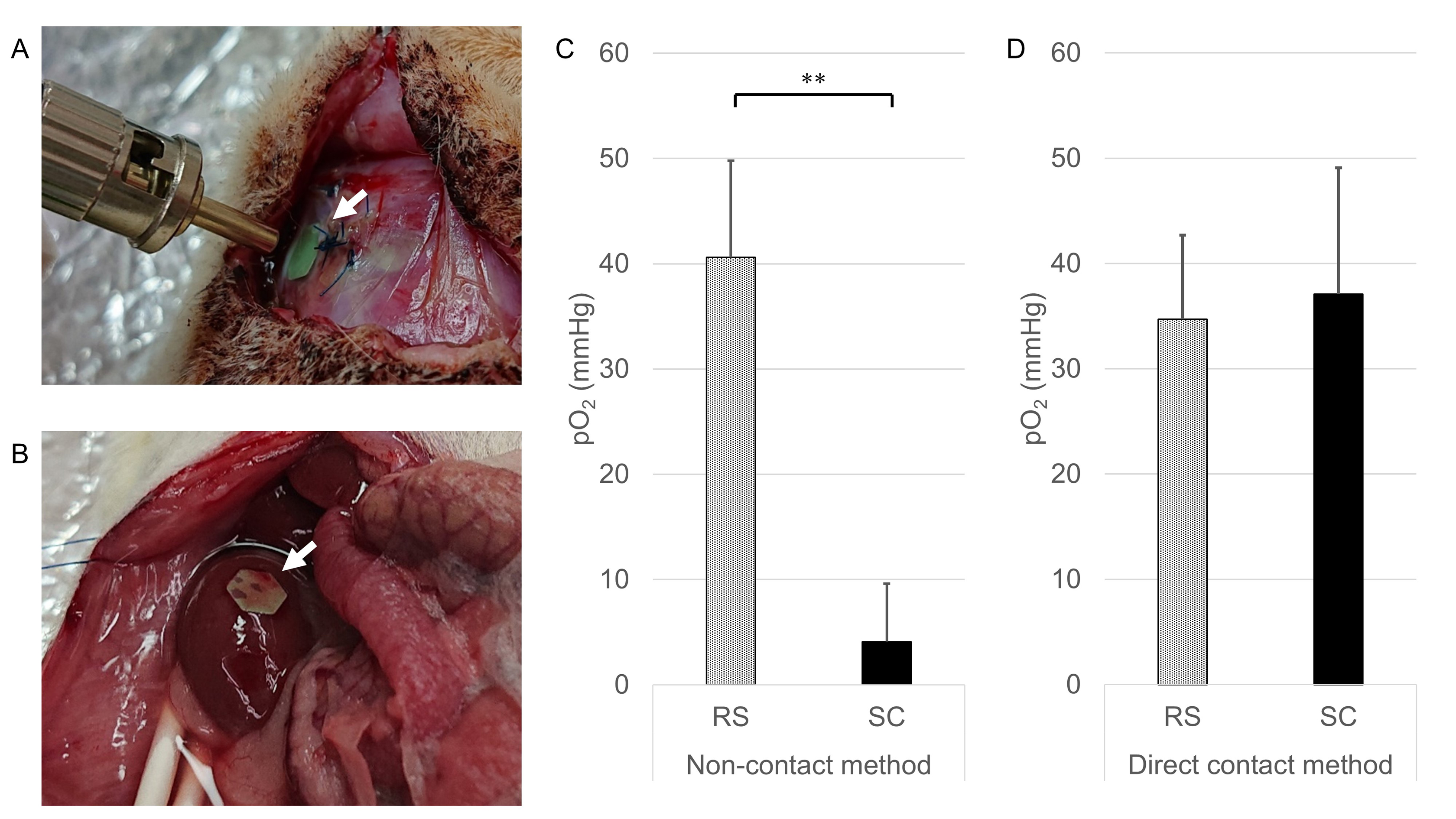
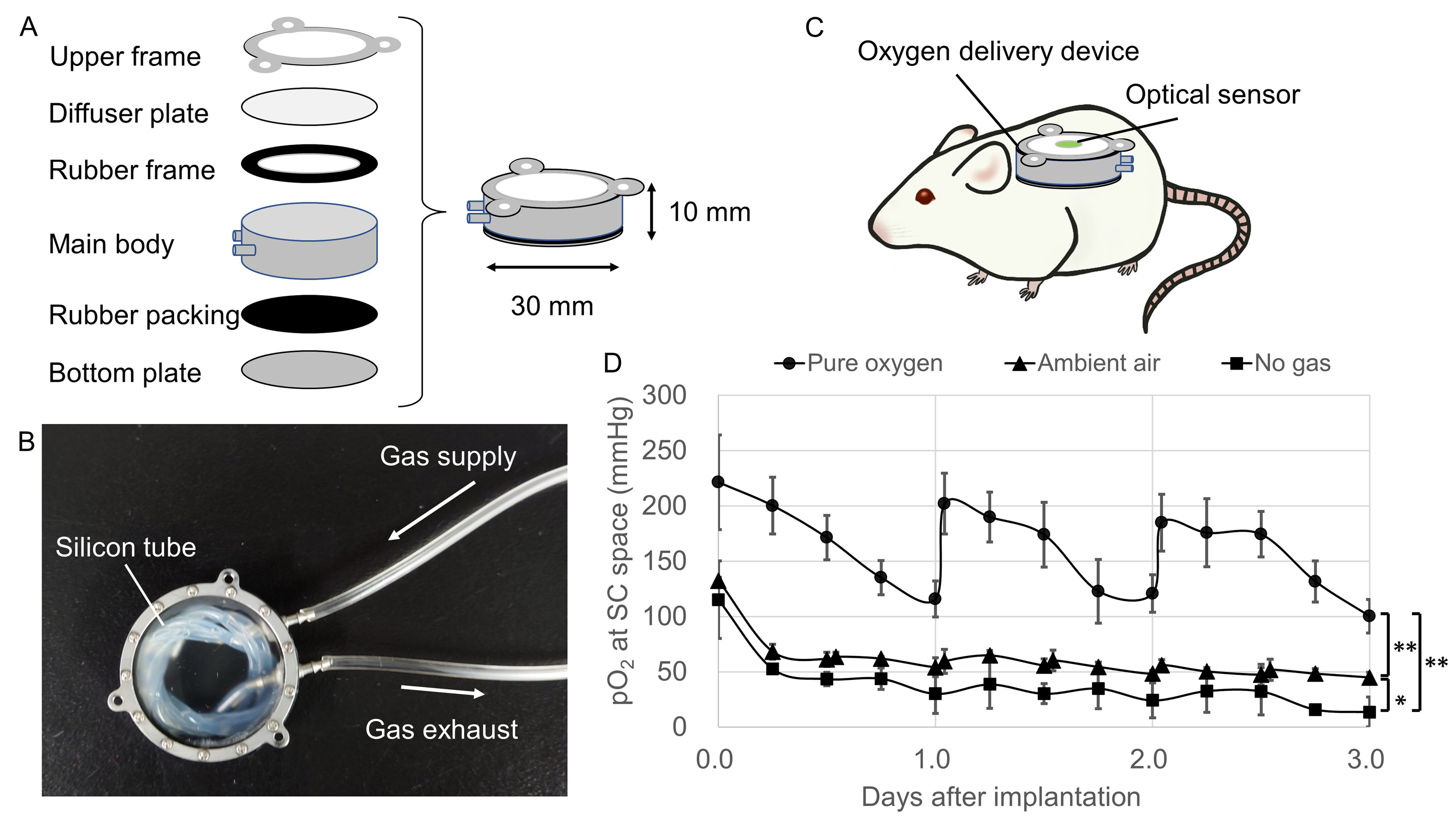
Method: An optical oxygen sensor was implanted into the SC (Fig.1A) and RS (Fig.1B) of rats. The pO2 values were measured by non-contact method using a contactless optical fiber at each site and compared to the values by direct contact method using a puncture-type optical oxygen sensor. We developed the oxygen delivery device and implanted it into rats subcutaneously to increase the pO2 of the SC (Fig.2A-C). Pure oxygen, Ambient air and No gas groups were made by the difference of oxygenation conditions of the oxygen delivery device. To verify the utility of this method, we transplanted syngeneic rat islets subcutaneously into diabetic recipients under several oxygenation conditions using the oxygen delivery device, then estimated the graft function by glucose tolerance test, and the graft viability by measuring the insulin extraction and the ATP/DNA ratio.
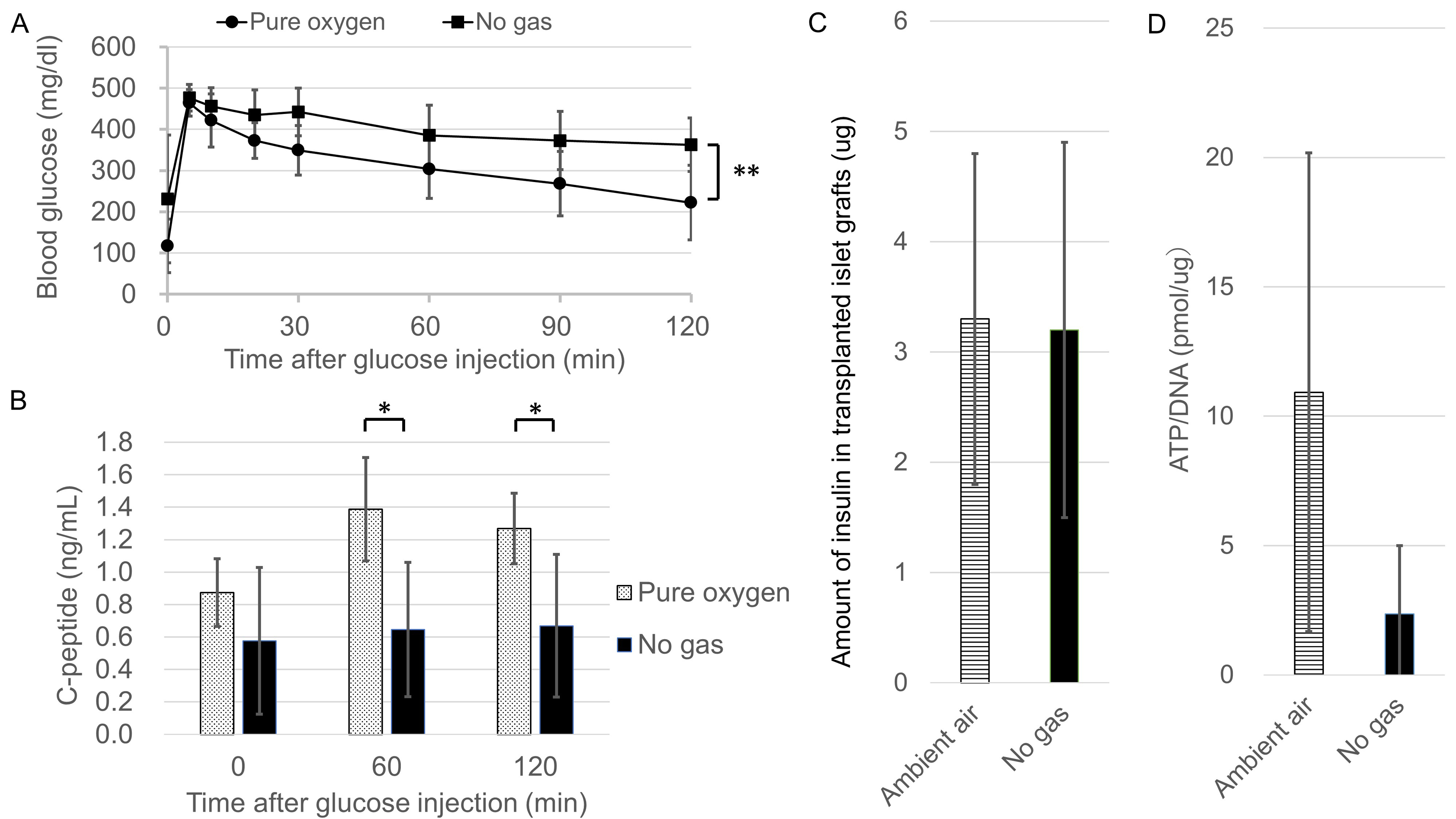
Results: In the non-contact method, the pO2 of the SC was significantly lower than that of the RS (Fig.1C, p<0.01). In contrast, no difference was observed in the direct contact method (Fig.1D, p=0.62). The pO2 of the SC was significantly increased in the Pure oxygen and the Ambient air groups compared to the No gas group (Fig.2D). In the Pure oxygen group, the glucose tolerance of the transplanted recipients was better (Fig.3A, p<0.01) and the serum C-peptide levels increased significantly (Fig.3B, p<0.05) in comparison to the No gas group, suggesting that superior functional engraftment of the islet grafts was seen in the Pure oxygen group. In terms of the amount of insulin in the islet grafts, there was no significant difference between the Ambient air and No gas groups (Fig.3C, p=0.81). However, the ATP/DNA of islet grafts in the Ambient air group tended to be higher than that in the No gas group (Fig.3D, p=0.07)
Conclusion: The transplanted islet function was correlated with the level of the pO2 of the SC and the optical sensor system was validated by correlating the pO2 values with the transplanted islet function. This novel technique revealed that islet viability was more damaged by hypoxic conditions at the SC than expected, indicating that islet engraftment may substantially improve if the pO2 levels reach those of the RS. Further refinements for a hypoxic condition using the present technique may contribute to improving the efficiency of subcutaneous islet transplantation.
[1] Komatsu, H.et al. Pancreas. 2018; 47, 533-543.
[2] Barkai, U. et al. Cell Transplant. 2013; 22, 1463-1476.
[3] Evron, Y. et al. Sci. Rep. 2018; 8, 6508.
[4] Komatsu, H. et al. Am. J. Transplant. 2018; 18, 832-842.
[5] Komatsu, H. et al. Biofabrication. 2018; 11, 015011.
[6] Bochenek, M. A. et al. Nat Biomed Eng 2. 2018; 810-821.
[7] Einstein, S. A. et al. Tissue Eng Part C Methods. 2016; 22, 1009-1017