Impact of GAD65 and IA2 autoantibodies on islet allograft survival
Joana Lemos1, Raffaella Pogiolli1, David Baidal1, Ana Alvarez1, Camillo Ricordi1, Rodolfo Alejandro1.
1Clinical Cell Transplant Program - Diabetes Research Institute (DRI), University of Miami, Miami, FL, United States
Introduction: Islet autoantibodies have a role in the pathogenesis of T1D and they serve as markers of beta-cell autoimmunity. Islet transplantation (ITx) shows promise in treating T1D, but the role of islet autoantibodies on graft survival has not been clearly elucidated. We aimed to analyze the effect of GAD65 and IA2 autoantibody positivity on islet graft survival and on to the attainment of insulin independence in T1D ITx recipients.
Method: A retrospective cohort of 47 ITx recipients was analyzed from 2000 to 2018 at our institution. Forty-four subjects received islets via intrahepatic portal infusion and 3 subjects into the omentum via laparoscopic approach. Induction immunosuppression consisted of anti-IL2 receptor antibody, anti-TNF and maintenance with dual combination of either sirolimus, tacrolimus or mycophenolate mofetil (Edmonton-like) in 38 subjects (80.9%). Nine (19%) subjects received induction with T-cell depletion and same maintenance. GAD65 and IA2 autoantibodies were analyzed pre-transplant and monthly post-transplant up to graft failure and categorized as follows: persistently negative, persistently positive, or seroconverters. Graft survival (c-peptide >= 0.5ng/mL) between groups was analyzed through U-Mann-Whitney test. Quade's nonparametric ANCOVA was used for adjustment of confounders. Gain of insulin independence was analyzed by Kaplan-Meier and Log-Rank tests. P value <0.05 was considered statistically significant.
Results: Subjects baseline characteristics are shown in table 1 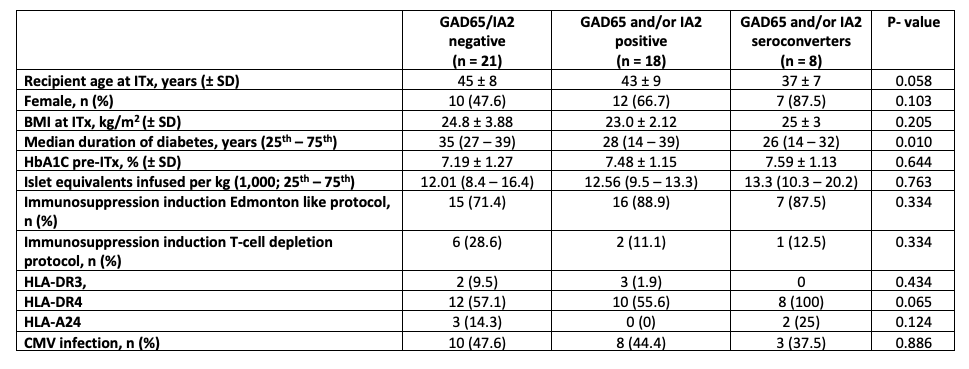
ITx recipients with persistent autoantibody negativity (n = 21) had longer graft function (98 [61 – 182] months;) compared to recipients with persistent autoantibody positivity (n = 18), even after adjustment for immunosuppressive induction protocol (P = 0.027).
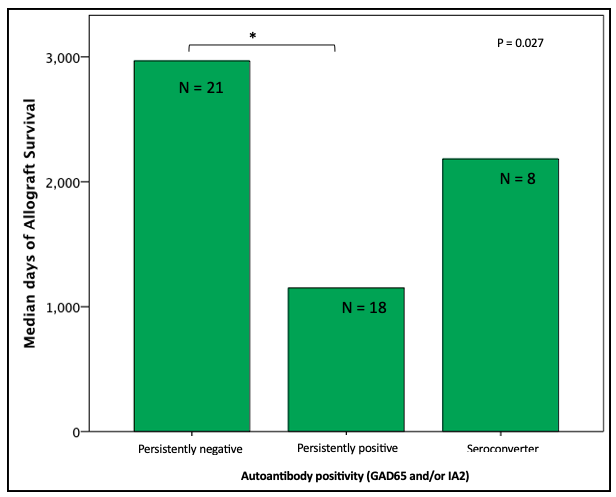
Graft survival time was shorter in the seroconverter group compared to the persistently negative group, but not statistically significant. Subjects persistently positive for GAD65 only (n = 8) had shorter graft survival compared to negative GAD65 only subjects (n = 21; P = 0.016). There was no difference in time of graft survival in subjects according to IA2 only positivity. The proportion of attainment of insulin independence was similar independent of autoantibody status.
Conclusion: Persistent autoantibody positivity to GAD65 and/or IA2 reduced the time of islet graft survival in our cohort. The presence of islet autoantibodies, as markers of persistent autoimmunity, may represent an underappreciated contributing factor to the failure of transplanted beta cells. Whether induction with T-cell depletion may lead to improved survival independent of islet autoantibody status could not be evaluated in our cohort. Larger prospective studies are needed to further address the role of islet autoantibody status on islet graft survival.
This study was supported by NIH grants R01 DK55347, R01 DK056953, R01 DK025802, DK070460, U42 RR016603, M01RR16587, UL1TR000460; the Miami Clinical and Translational Science Institute (CTSI) from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities; the Juvenile Diabetes Research Foundation International 4-200-946, 4-2004-361, 17-2012-361, 3-SRA-2017-347-M-B; the State of Florida; and the Diabetes Research Institute Foundation. The authors are grateful to the members of the cGMP Human Cell Processing Facility, the pre-clinical Human Immunology and Immunogenetics Program, the Clinical Cell Transplant Program (CCTP) at the Diabetes Research Institute (DRI), and the University of Miami CTSI for their support of this work. .
[1] Vendrame F, Pileggi A, Laughlin E, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. Apr 2010;59(4):947-57. doi:10.2337/db09-0498
[2] Froud T, Ricordi C, Baidal DA, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. Aug 2005;5(8):2037-46. doi:10.1111/j.1600-6143.2005.00957.x
[3] Burke GW, Vendrame F, Pileggi A, Ciancio G, Reijonen H, Pugliese A. Recurrence of autoimmunity following pancreas transplantation. Curr Diab Rep. Oct 2011;11(5):413-9. doi:10.1007/s11892-011-0206-y