Biological equivalence of genetically engineered porcine pericardium for biological heart valve manufacture: a randomized 150 day blind study in sheep.
Christopher G McGregor1,2, Jacob Salmonsmith2, Gaetano Burriesci2,3, Guerard Byrne1,2.
1Department of Surgery, University of Minnesota, Twin Cities, Minneapolis, MN, United States; 2Institute of Cardiovascular Science, University College London, London, United Kingdom; 3Bioengineering Group, Ri.MED Foundation, Palermo, Italy
Introduction: Current biological heart valves (BHVs) contain the porcine antigen alpha-Gal (Gal). The presence of this antigen creates a clinical immune disparity in heart valve recipients since humans universally produce anti-Gal antibody. We have shown that human anti-Gal antibody causes an immune inflammation in fixed bioprosthetic tissue resulting in accelerated tissue calcification (Cal) of Gal+ wild type tissue but not Gal-free tissue (GalKO). Sourcing BHV tissue from GalKO animals eliminates this immune disparity, potentially mitigating BHV Cal and extending device durability. This study tests ISO standard biological equivalence (BE) of valves made from GalKO pig pericardium (PP) to current BHV materials.
Method: This randomized blind study of mitral valve implantation in adolescent sheep with a 150-day follow-up tested BE in 2 groups: A) (n=6) BHVs made from GalKO PP and B) BHVs made from current materials: commercial bovine pericardial BHVs (n=2, Carpentier-Edwards Perimount Magna Mitral Ease), commercial porcine BHVs ( n=2, Abbott Epic porcine tissue valves) or standard PP (n=2) (n=6 in total) (Figure 1). Porcine pericardial BHVs (PP-BHVs) from WT or GalKO tissue were fixed in 0.6% glutaraldehyde and post treated with 4% formaldehyde, 22% ethanol and 1.2% Tween (FET). Hemodynamic performance was measured every 30-days and at explant. Explant histology was blindly scored for pannus formation, fibrin deposition and inflammation. Tissue Cal was measured by atomic spectroscopy.
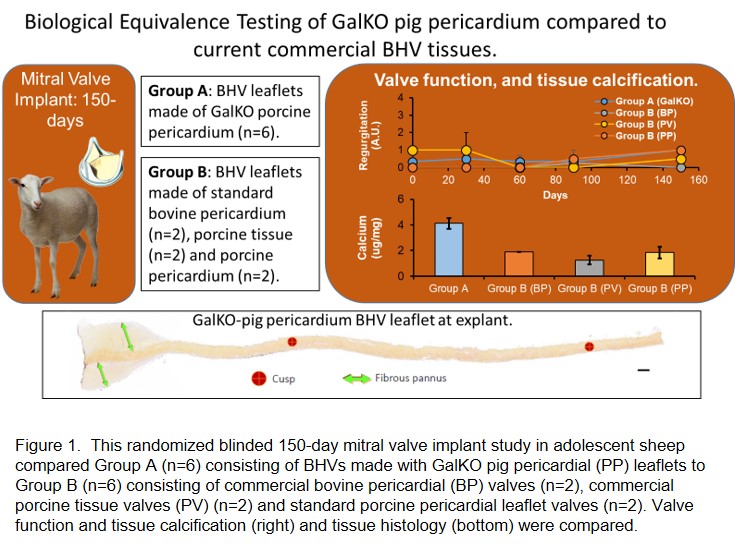
Results: All BHV recipients were healthy throughout the study and completed the 150-day postoperative period. There were no adverse valve-related events during this study. All animals demonstrated good hemodynamic performance throughout the study. Recipient sheep showed no clinically relevant abnormal hematology or blood chemistry, with no differences between the study groups. There was no apparent difference between the study groups for echocardiographic hemodynamic performance measured on day 0, 30, 90, and 150 post valve implant. There was no meaningful differences in regurgitation, which was minimal, or reduction in leaflet motion or coaptation observed for any of the valves throughout the study. The histology of Group A and B cusps at explant were similar and within the range of expected findings for a BHV after 150 day implantation in the ovine model. Atomic spectroscopy detected similar and low levels of tissue Cal in all valves (range 1.6 - 4.1 ug/mg), within the lower end of historical equivalent studies (1.0 - 72.0 ug/mg).
Conclusions: This study demonstrates BE of GalKO PP valves to current BHVs. Use of GalKO pigs for commercial surgical or transcatheter BHV manufacture could eliminate anti-Gal induced Cal and increase BHV durability.
This work was funded by a Medical Research Council Development Pathway Funding Scheme (MR/R006393) to CGM..