A systematic scoping review of studies investigating the fate of cells administered in clinical and whole organ studies
Madison Cooper1, Aaron J Buhagiar1, Lucilia Mouries2, Stephen Patrick3, Mya S Thu4, Vladimir Ponomarev5, Lucy Bates1, Colin Wilson1, Neil Sheerin1, William E Scott III1.
1Translational and Clinical Research Institute, Newcastle University, Newcastle University, United Kingdom; 2Senior Scientific Program Manager, Health & Environmental Sciences Institute (HESI), Washington DC, DC, United States; 3University College London, London, United Kingdom; 4Co-Founder and CEO, Visicell Medical Inc., San Diego, CA, United States; 5Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Introduction: Cellular therapy has promising applications in regenerative medicine. Despite this, little is known about the distribution and fate of these cells. The literature documents multiple approaches to track cells using labels and various modalities such as; MRI, PET, SPECT and Scintigraphy, however, it is scattered and the terminology inconsistent. To address this, HESI CT/TRACS committee established the Data Portal for Imaging Cell Therapies (DPICT) database. An initial attempt to populate the database by academic, regulatory, and industry experts identified 61 articles though many studies were likely missed. To address this, we performed a systematic scoping review to collate all the methods of tracking cells that have been used in clinical trials and human whole organ studies where cells are administered in vivo as a therapeutic. This abstract describes the establishment of an iterative pilot search to investigate the articles retrieved before searching Embase, Cochrane, Scopus and CINAHL databases.
Method: The initial DPICT database publications were used as a positive control. We searched the title, abstract and key words of articles published in English, post-2001 in the Medline Ovid database with the following key word sequence (figure 1) structure: (imaging modality) AND (cell label) AND (cell type) AND (humans OR human study OR human studies OR clinical study OR clinical studies OR clinically OR patient* OR perfusion).

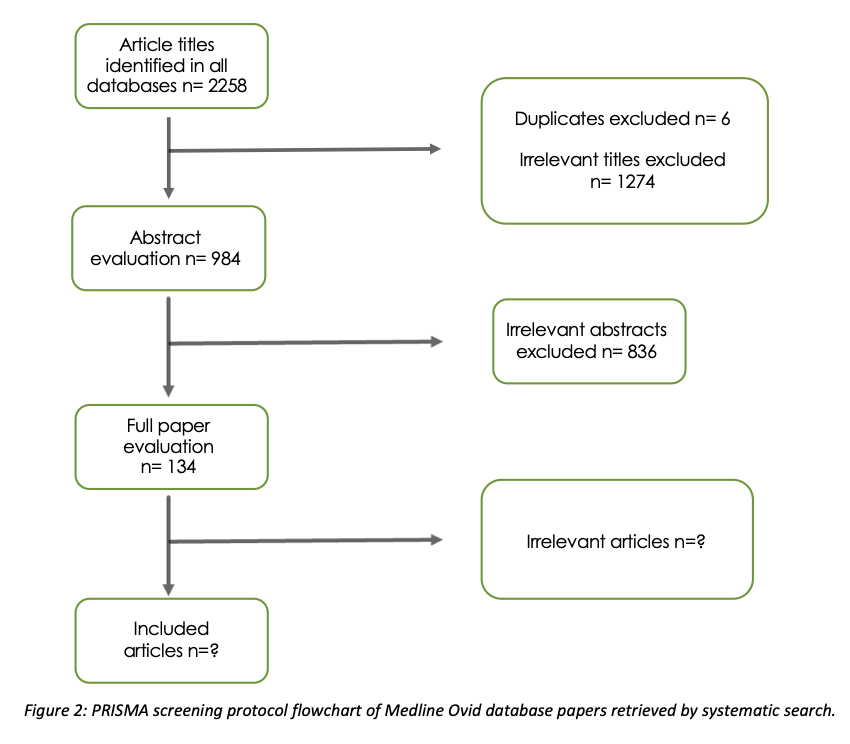
Results: Of 2264 articles identified, only 134 full papers will be included in this review, following screening and eligibility assessment (figure 2). Of the 837 abstracts discarded, the majority of papers were evaluating the safety/efficacy of a label or modality (33%) or of cellular therapies (14%). Preclinical studies (15%) tracking cell fate, occurred in canine (0.1%), hamster (0.1%), horse (0.6%), mouse (6%), pig (1%), rabbit (0.5%), rat (3%) and sheep (0.4%).
Discussion: We created a ‘clinical trials filter’ for the search as the Medline Ovid filters excluded relevant papers. This filter can streamline the population of the DPICT database clearly identifying how this repository of cell tracking articles has been cultivated to benefit the cell-therapy community. This will guide future research by identifying gaps in the literature and signposting users to relevant studies which may benefit their application.
This study is funded by the National Institute for Health and Care Research (NIHR) Blood and Transplant Research Unit in Organ Donation and Transplantation (NIHR203332), a partnership between NHS Blood and Transplant, University of Cambridge and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NIHR, NHS Blood and Transplant or the Department of Health and Social Care.. This study is funded by the Health and Environmental Science Institute (HESI). The 'HESI' is organized and operated as a nonprofit, charitable and scientific organization. .