Agustina Forgioni, Japan has been granted the IPITA Congress Scientific Award
Efficacy of ex-vivo generated donor antigen-specific immunomodulatory cells on pancreatic islet transplantation
Agustina Forgioni1, Masaaki Watanabe2, Ryoichi Goto1, Takuya Harada1, Takuji Ota1, Tsuyoshi Shimamura3, Akinobu Taketomi1.
1Department of Gastroenterological Surgery I, Hokkaido University, Sapporo, Japan; 2Department of Transplant Surgery, Hokkaido University, Sapporo, Japan; 3Department of Organ Transplantation Medicine, Hokkaido University Hospital, Sapporo, Japan
Introduction: Pancreatic islet transplantation (PITx) is a promising treatment option for patients with type 1 diabetes mellitus (DM). Controlling the innate immune response is essential not only to prevent the rapid destruction of transplanted islets, but also for the long-term acceptance of allogeneic islet grafts. We have previously demonstrated that the adoptive transfer of donor antigen-specific immunomodulatory cells (IMCs) could induce tolerance in recipient patients after living-donor liver transplantation. We hypothesize that the same beneficial effects could be applied to PITx.
Methods: Based on our clinical trial, IMCs were generated by co-culturing C57BL/6 (H-2b) mouse splenocytes with irradiated BALB/c (H-2d) splenocytes in the presence of anti-CD80/86 monoclonal antibodies (mAbs). The phenotypes of IMCs were assessed by flow cytometry. The immunosuppressive effect was evaluated by mixed lymphocyte reaction (MLR). C57BL/6 splenocytes were stained with CellTrace Violet (CTV) and stimulated with irradiated donor (BALB/c) or 3rd party (C3H/HeJ, H-2k) splenocytes and the proliferation of CTV-labelled cells was measured by flow cytometry. To evaluate the anti-inflammatory potential of IMCs, RAW264 cells (5x105 cells/well) were stimulated with lipopolysaccharide (LPS) with or without IMCs for 24 hours. The expressions of CD80 and CD86 were assessed by flow cytometry, and the concentration of nitrite in the supernatant was also measured by Griess assay. Pancreatic islets from C57BL/6 mice (250 islets) were transplanted into the liver via the portal vein of STZ-induced C57BL/6 DM mice. In this PITx setting, IMCs were administered intraportaly, intravenously, or intraperitoneally, and the liver samples were collected 12 hours after PITx for pathological evaluation and measurement of mRNA expression of pro-inflammatory cytokines.
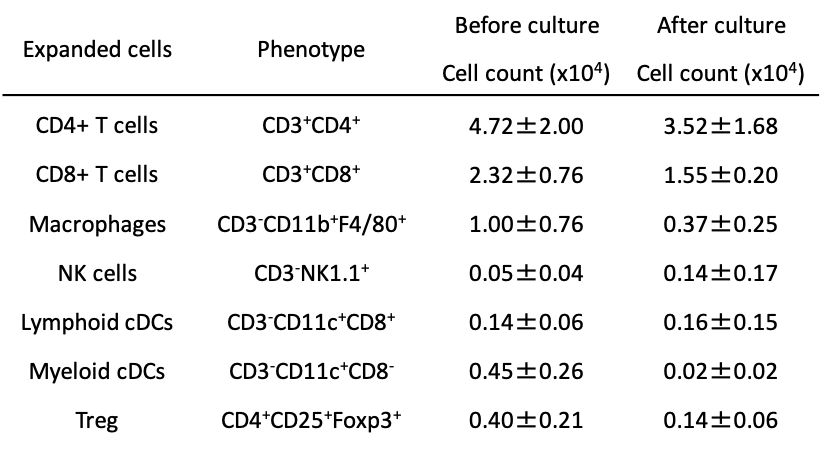
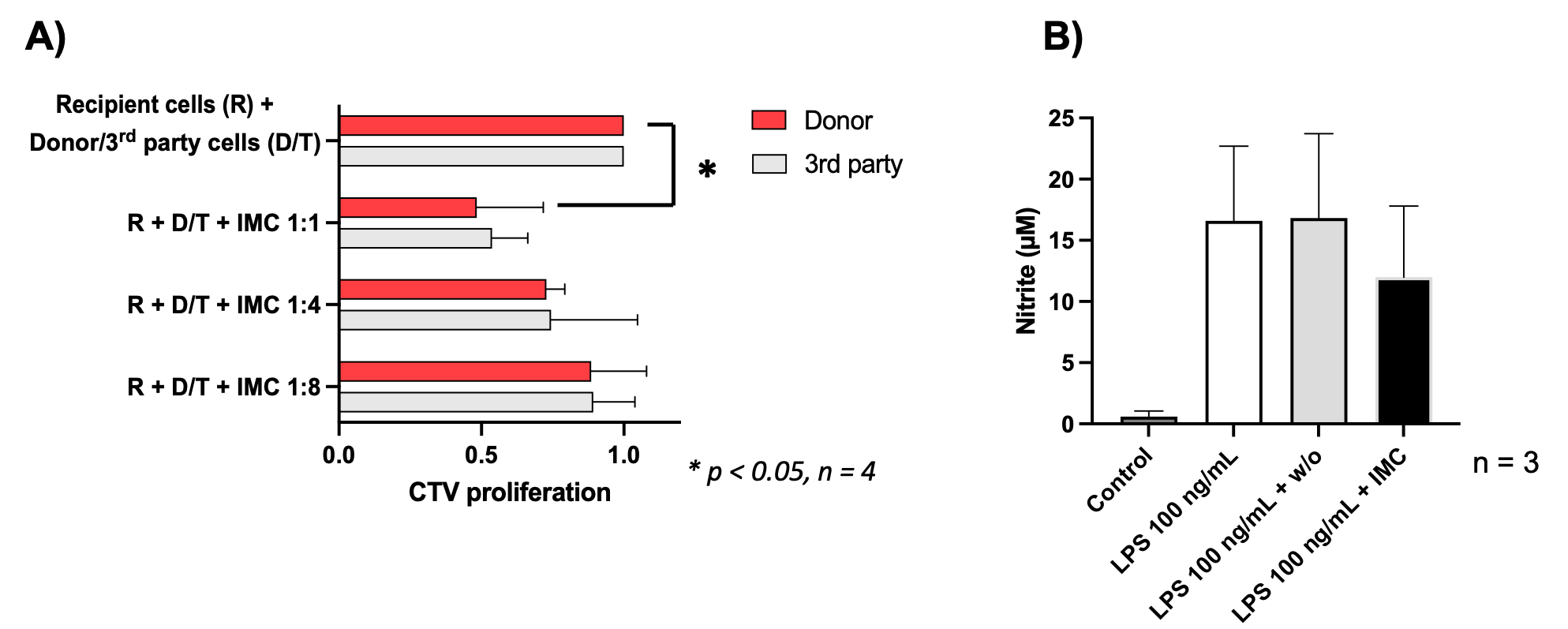
Results: During the generation of IMCs, the cell number decreased from 80x106 to 8.3±3.9x106 (mAbs group) and to 6.5±3.7x106 cells (w/o mAbs group) (mean±SD, n=4). The phenotypes of IMCs were evaluated by flow cytometry (Table 1). IMCs effectively impeded the proliferative response of splenocytes from C57BL/6 mice against BALB/c antigen, not against 3rd party antigen, in an IMCs-dose-dependent fashion (Fig. 1. A). The treatment of RAW264 cells with IMCs at the time of LPS stimulation reduced nitric oxide production compared to control groups (Fig. 1 B). The expression of CD80 and CD86 was inhibited in the IMCs-treated group. IMCs could be safely injected together with pancreatic islets into DM recipient animals. However, the mRNA expressions of TNF-α, IL-1b, MCP-1, IL-6, and MIP-1b in the liver and markers of liver damage were upregulated especially in the intra-portal injected animals.
Conclusion: IMCs showed both immunosuppressive and anti-inflammatory properties and could be safely injected into DM recipient mice. The strategy using IMCs may be a promising therapeutic approach to improve the outcome of PITx.
[1] Gustafson E, et al. The Instant Blood-Mediated Inflammatory Reaction Characterized in Hepatocyte Transplantation. Transplantation. 2011; 91: 632–8.
[2] Todo S, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016; 64(2): 632–43.
[3] Watanabe M, et al. Ex Vivo Generation of Donor Antigen-Specific Immunomodulatory Cells: A Comparison Study of Anti-CD80/86 mAbs and CTLA4-lg Costimulatory Blockade. Cell Transplant. 2018; 27(11): 1692–704.
[4] Watanabe M, et al. Efficacy of DHMEQ, a NF-κB inhibitor, in islet transplantation: II. Induction DHMEQ treatment ameliorates subsequent alloimmune responses and permits long-term islet allograft acceptance. Transplantation. 2013; 96(5): 454–62.