Gal knockout sheep: a new paradigm for future preclinical biological heart valve testing.
Christopher GA McGregor1,2, Guerard W Byrne1,2, Zhiqiang Fan3, Ying Liu3, Misha Regouski3, Irina A Polejaeva3.
1Department of Surgery, University of Minnesota, Twin Cities, Minneapolis, MN, United States; 2Institute of Cardiovascular Sciences, University College London, London, United Kingdom; 3Department of Animal, Diary and Veterinary Sciences, Utah State University, Logan, UT, United States
Introduction: There is currently a clinical discordance between patients who universally produce anti-Gal antibody and commercial biological heart valves (BHVs) which express the major xenogeneic antigen, galactose α1,3 galactose (Gal). We and others have shown that human antibody to Gal and other known xenogeneic glycans accelerates calcification of fixed bioprosthetic tissue and thereby contributes to BHV structural valve degeneration. BHV implantation in juvenile sheep, the accepted model for regulatory approval of new BHVs, is performed to demonstrate biocompatibility and physiological performance. This standard model does not however detect the immunological incompatibility that currently exists because standard sheep produce Gal antigen and do not produce anti-Gal antibody. The objective of this research was to develop a genetically engineered (GE) sheep suitable for pre-clinical testing, which, like humans, produces cytotoxic anti-Gal antibody.
Methods: PCR primers were designed according to sheep GGTA1 genome sequences (GenBank, NC_040254.1) to amplify exon 4 and partial intron flanking sequences (522bp). Exon 4 contains the translation start signal. Sheep fetal fibroblasts (SFFs) were transfected by electroporation with ribonuclear complexes containing Cas9 protein and an exon 4 specific gRNA, (Figure 1A). Single-cell-derived transfected SFF colonies were isolated by limiting dilution and screened by PCR and Sanger sequencing for biallelic mutations (Figure 1B). Male and female GGTA1-/- SFF colonies were used as donor cells for somatic cell nuclear transfer. Peripheral blood mononuclear cells (PBMCs) from cloned sheep were screened for Gal expression and serial serum samples were assayed by ELISA for anti-Gal antibody. Sheep antibody binding and cytotoxicity was assessed by flow cytometry using HEK cells expressing porcine GGTA1.
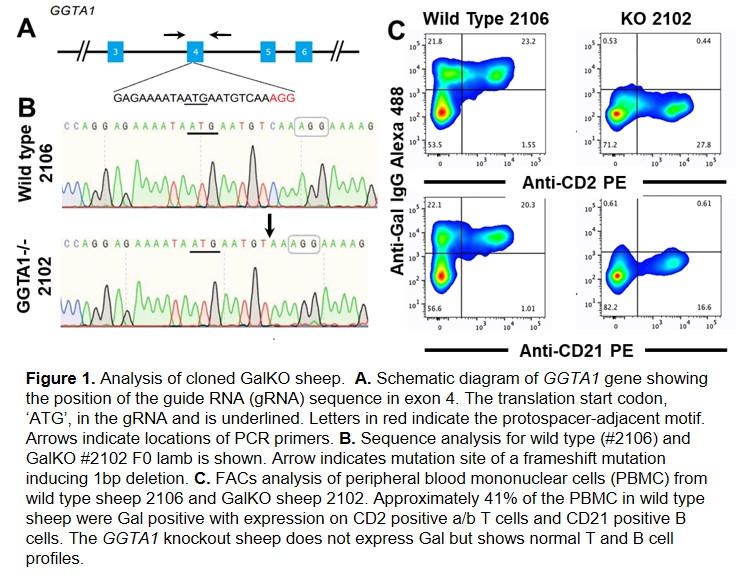
Results: To date 22 pregnancies carrying male and female GGTA1-/- SFF have been established and two GGTA1-/- sheep (Gal-KO) have been analyzed. Gal-KO sheep are devoid of the Gal antigen (Figure 1C) and begin expressing cytotoxic anti-Gal antibody by 2 - 3 months of age which increased to clinically relevant levels by 6 months of age (Figure 2).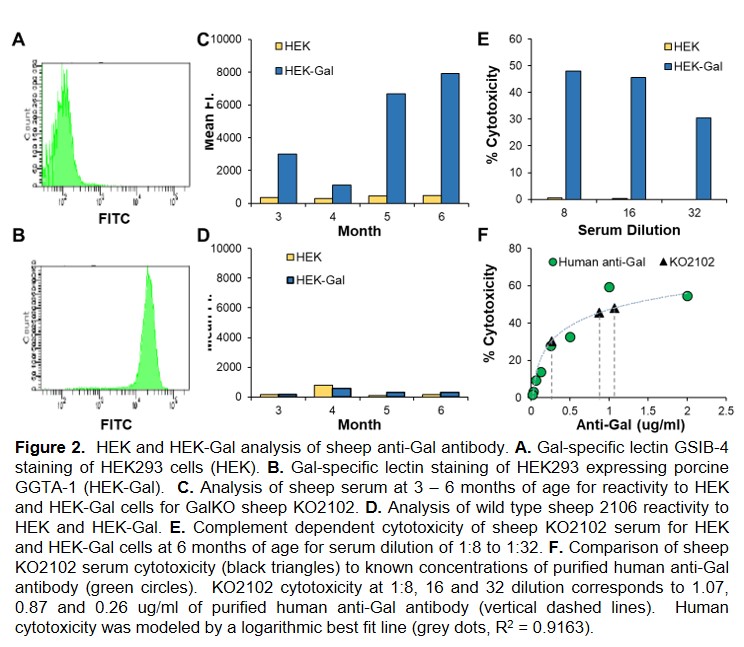
Conclusions: GalKO sheep represent a new standard for preclinical testing of BHVs (surgical or transcatheter) and other innovative emerging regenerative tissue technologies because they can, for the first time, account for human immune responses to the major residual xenogeneic antigen Gal which persists after current BHV tissue processing. This technology can, for the first time, be used to directly test the role of antibody in BHV calcification, identify earlier the consequences of this immune disparity and avoid unexpected clinical sequelae seen in the past. This development opens the door to rapid industry substitution of GE GalKO pigs for standard pigs, as a source of Cal-resistant valve tissue with the potential to transform the BHV field in a sustained way.
This work was funded by FIOS Therapeutics and by the US Department of Agriculture Multistate Project W-4171 to IAP.