Single nucleotide polymorphisms associated with human islet insulin secretion
Anjali Verma1, Steven Schrodi2, Domenico Bosco3, Georg Ehret4, Mark Keller5, Raphael Meier1.
1Department of Transplant and Surgery, School of Medicine, University of Maryland, Baltimore, MD, United States; 2Department of Medical Genetics, University of Wisconsin, Madison, WI, United States; 3Department of Surgery, Cell Isolation and Transplantation Center, Geneva University Hospitals and University of Geneva, Geneva, Switzerland; 4Cardiology, Department of Medical Specialties, Geneva University Hospitals and University of Geneva, Geneva, Switzerland; 5Department of Biochemistry, University of Wisconsin, Madison, WI, United States
Introduction: Allotransplantation of pancreatic islets is currently a successful treatment option for type 1 diabetic patients. Donor phenotype affects islet transplantation outcomes. However, genetic factor predisposing for diabetes and metabolic disease are typically not assessed in donors. We sought to investigate the potential role of donor genetic factors influencing insulin secretion.
Methods: We extracted the DNA of close to 200 pancreas donors and used the Cardio-MetaboChip to query the 196,725 single nucleotide polymorphisms (SNPs) associated with metabolic syndrome components. We used Glucose‐stimulated insulin secretion (GSIS) as main phenotypical variable to test our genotype dataset. We used islet graft survival as an additional phenotypical variable. We performed GSIS in INS-1 cells to test the potential insulin secretion molecules/genes identified.
Results: We identified several loci associated with donor insulin secretion in vitro (Figure 1). We identified three specific SNPs in the same linkage disequilibrium (LD), mapping upstream of CCL2 gene, which were associated with GSIS. When we mapped the ATAC seq peaks to this region, we found that open chromatin status varied between diabetic versus non-diabetic individuals. Further, motif analysis suggested that these SNPs could participate to the disruption of a binding site of NF-κB transcription factor, a regulator of CCL2 gene. To investigate further, we induced NF-κB pathway using TNF-α in INS-1 cells and confirmed a reduction in insulin in response to glucose upon TNF-α stimulation (Figure 2).
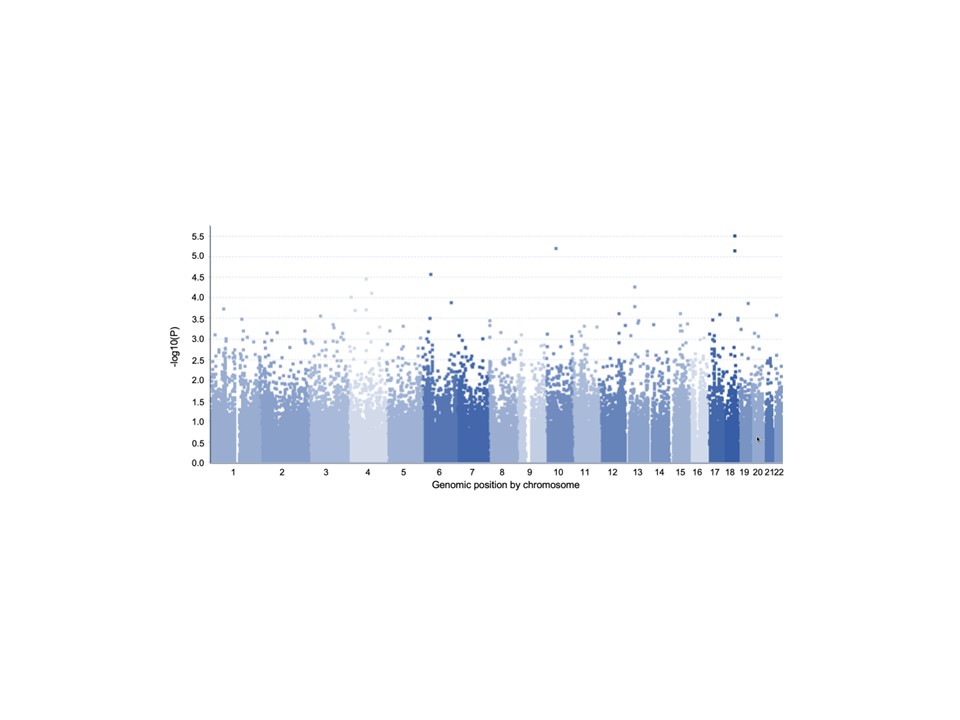
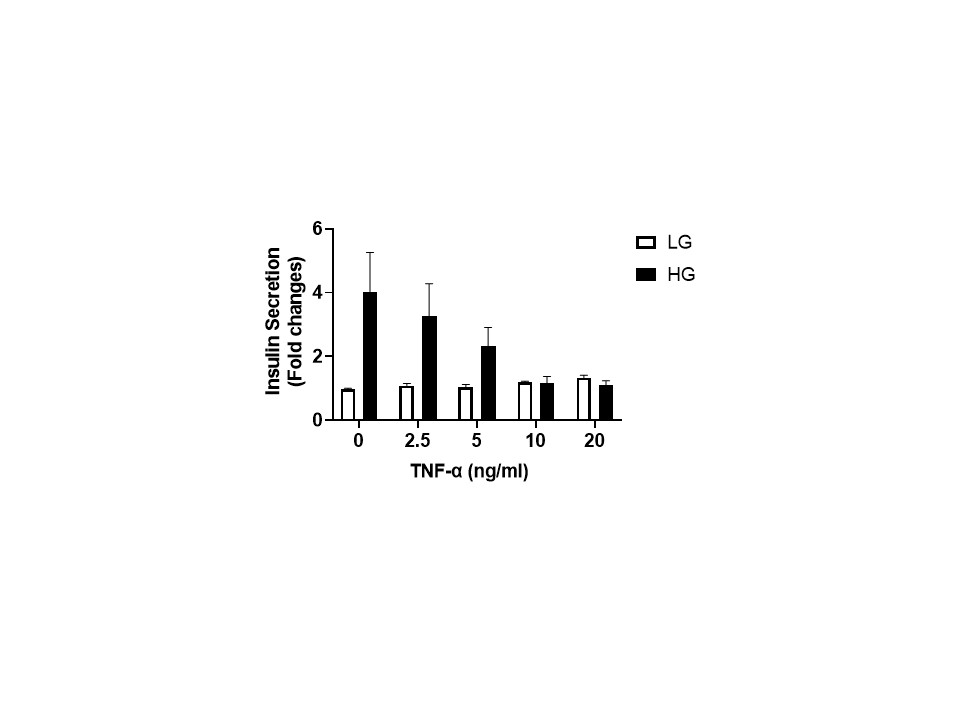
Conclusion: Our data suggest the potential involvement of an NF-κB binding site for CCL2 regulation and a potential role in glucose homeostasis. These observations could help identifying preclinical conditions with impaired insulin secretion and their potential impact on islet donor selection.
Diabetes Research Connection Grant.
[1] Niclauss, N., et al., Beta-Cell Replacement: Pancreas and Islet Cell Transplantation. Endocr Dev, 2016. 31: p. 146-62.
[2] Meier, R.P.H., et al., Pancreas preservation fluid microbial contamination is associated with poor islet isolation outcomes - a multi-centre cohort study. Transpl Int, 2018. 31(8): p. 917-929.
[3] Kim, H. E., et al., Tumour necrosis factor-alpha-induced glucose-stimulated insulin secretion inhibition in INS-1 cells is ascribed to a reduction of the glucose-stimulated Ca2+ influx." J Endocrinol, 2008.198(3): p.549-560.