Enhancing mesenchymal stem cell’s intrinsic expression of MMP-9 to augment their anti-fibrotic capacities for the treatment of liver diseases
Anjali Verma1, Shani Kamberi1, Magali Fontaine2, Civin Curt3, Daniel Maluf1, Valeria Mas1, Raphael Meier1.
1Department of Transplant and Surgery, University of Maryland, Baltimore, MD, United States; 2Department of Pathology, University of Maryland, Baltimore, MD, United States; 3Department of Physiology, University of Maryland, Baltimore, MD, United States
Introduction: Chronic liver insult leads to fibrosis, cirrhosis and liver failure. Liver transplantation is limited due to organ shortage. Alternatives such as cell therapies using mesenchymal stem cells (MSC) have been investigated. Matrix metalloproteinase-9 was found to be a key player in MSC antifibrogenic effect. We aimed to develop genetically modified MSCs with enhanced MMP-9 secretion to treat liver fibrosis.
Method: We used two different approaches to increase the expression of MMP-9 in MSCs. We used siRNA to knockdown regulator genes for key cytokines potentially responsible for human MSC anti-inflammatory effect. We also utilized the CRISPRa-dCas9 technology to increase the intrinsic MMP-9 gene expression in MSCs. We tested siRNA-modified MSC conditioned medium (CM) on LX-2 hepatic stellate cells (cell secreting collagen during liver fibrogenesis) and analyzed responses to profibrogenic stimuli by TGF-β1.
Results: We tested BCl3, Ets1, Smad4 and TGFβ1 knockdown. BCl3, Ets1, and TGFβ1 knockdown did not lead to significant anti-fibrotic cytokine upregulation. We achieved a five-fold enhancement in MMP-9 gene expression using Smad4 knockdown as quantified by qPCR analysis (Figure-1 A and B).
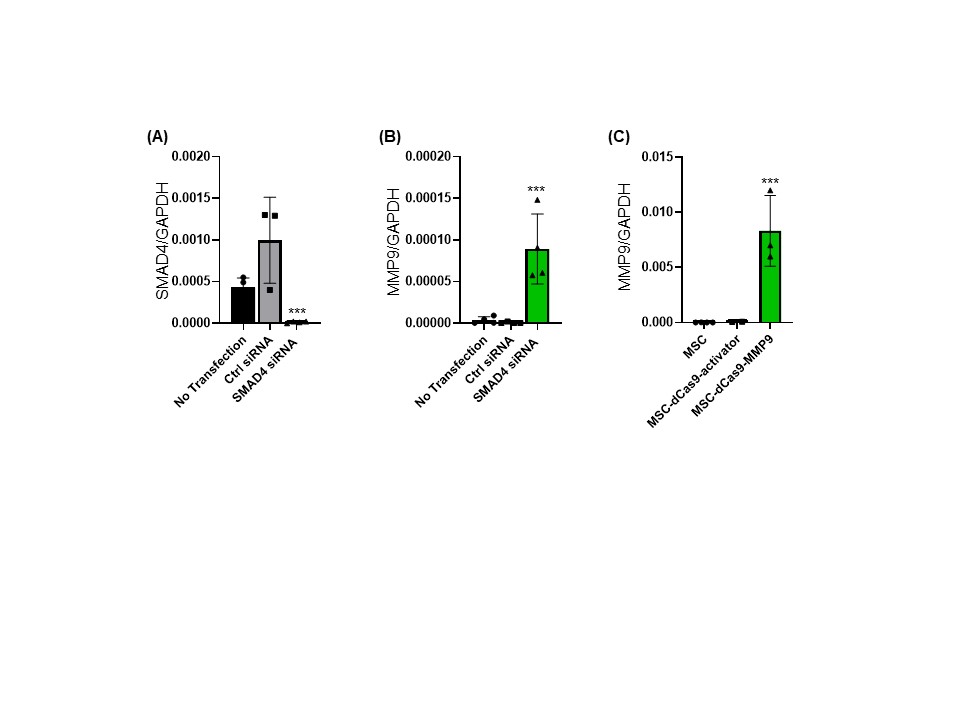
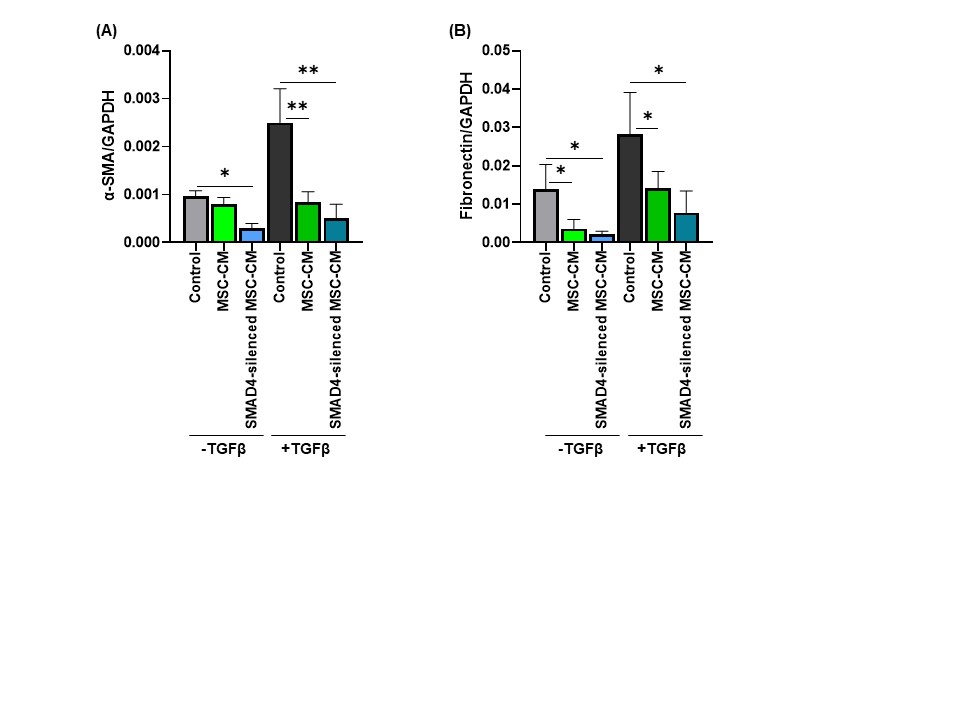
The increased levels of MMP-9 protein produced by MSC was confirmed using FACS and ELISA. We also generated the CRISPR-modified MSC with increased MMP-9 expression and observed approx. 120-fold increase in the MMP9 gene expression (Figure-1C). The conditioned media from SMAD4-silenced MSC significantly reduced hepatic stellate cells activation in vitro compared to the non-modified MSC conditioned medium and non-conditioned medium control. Furthermore, we observed that the SMAD4-silenced MSC-CM reduced the levels of the liver fibrosis markers such as α-SMA, and fibronectin (Figure-2 A and B), collagen type-I, and MMP-2 as compared to the non-modified MSC-CM and non-conditioned medium control in LX-2 cells.
Conclusions: Our data confirms the key role of MMP-9 as an antifibrotic mediator responsible for MSC effect. MMP-9 secretion enhancement via SMAD4 knockdown and CRISPRa-dCas9 technology has the potential to boost MSC therapeutic effect in liver fibrosis.
Lisa Dean Moseley Foundation. Maryland Stem Cells Research Foundation.
[1] Koyama, Y. and D.A. Brenner, Liver inflammation and fibrosis. J Clin Invest, 2017. 127(1): p. 55-64.
[2] Meier, R.P., et al., Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol, 2015. 62(3): p. 634-41.
[3] Higashiyama, R., et al., Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology, 2007. 45(1): p. 213-22.