Emerging data from a study of tegoprubart for the prevention of rejection in kidney transplant recipients: Implications for a similar study in islet cell transplantation
John Gill3, Patrick Coates6, Jean Tchervenkov4, Jeffrey Bornstein1, Paul Harden5, Matthew Kadatz3, Piotr Witkowski2.
1Eledon Pharmaceuticals, Irvine, CA, United States; 2Surgery, University of Chicago, Chicago, IL, United States; 3Nephrology, University of British Columbia, Vancouver, BC, Canada; 4Surgery, McGill University, Montreal, QC, Canada; 5Nephrology, Oxford, Oxford, United Kingdom; 6Nephrology, Royal Adelaide, Adelaide, Australia
Introduction: Islet cell transplantation has the potential to be a functional cure for type 1 diabetes, but this option is infrequently used, in part because of the untoward effects of one of the anti-rejection medications, tacrolimus, which can be toxic to the implanted cells, necessitating multiple transplants. Tegoprubart is a monoclonal antibody directed against the CD40 ligand (CD40L), a key mediator of co-stimulation. Inhibition of CD40L should result in a decrease in both cell and antibody mediated immunity and create a more tolerogenic immune environment. Importantly, tegoprubart is not toxic to islet cells. Tegoprubart has been shown to be effective in animal models of both islet cell and kidney transplantation and is currently being studied in kidney transplant recipients.
Methods: In the ongoing kidney transplantation study, up to 12 adults receiving a kidney transplant from either a living or deceased donor will be enrolled. To be eligible, this must be their first transplant, they must be seropositive for EBV, free of donor specific antigens, have low panel reactive antibodies, and the organ cannot be from an extended criteria donor or have a prolonged cold ischemia time. All participants will receive rATG induction and a maintenance regimen consisting of tegoprubart 20 mg/kg IV administered every 3 weeks after an initial loading regimen, mycophenolate and corticosteroids. Enrollment of the first 4 participants is staggered such that the Data Monitoring Committee (DMC) needs to review the data from the first 28 days on study of the preceding participant before the next participant can be enrolled. Participants will remain on study for a year, after which time they will have the option of continuing tegoprubart in an extension study. The primary endpoint is safety. Secondary endpoints include characterizing the pharmacokinetic profile of tegoprubart, the incidence of biopsy proven rejection (BPAR), changes in estimated glomerular filtration rate (eGFR) and exploratory biomarkers including donor derived cell free DNA.
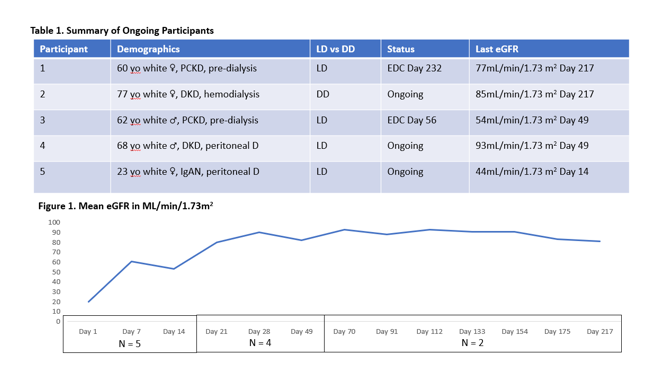
Results: The study is ongoing. As of the abstract submission deadline, May 2023, 5 participants have been transplanted, and 3 are ongoing. No participant has experienced rejection. One discontinued due to an SAE of BK viremia and another for mild alopecia and fatigue. BK viremia was the only SAE reported to date, and the drug appears safe and well tolerated. Participant information is summarized in Table 1, and mean eGFR is summarized in Figure 1.
Conclusions: Data to date from this calcineurin free study are encouraging, with no rejections, a good safety profile and excellent allograft function. These data support studying tegoprubart in a population of islet cell transplant recipients to assess whether this will improve outcomes in this setting. Such a study is planned by investigators at the University of Chicago.