Islet-alone vs. islet-after-kidney transplantation glycemic outcomes: Report from the Collaborative Islet Transplant Registry
David Baidal1, Cassandra Ballou2, Lawrence G Hunsicker3, Thomas L Eggerman4, Rodolfo Alejandro1, Michael Rickels5.
1Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, Diabetes Research Institute, University of Miami, Miami, FL, United States; 2Collaborative Islet Transplant Registry Coordinating Center, The EMMES Company LLC, Rockville, MD, United States; 3Clinical Trials Statistical and Data Management Center, University of Iowa, Iowa City, IA, United States; 4National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, United States; 5Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Introduction: Reported rates of long-term glycemic outcomes from the Clinical Islet Transplantation Consortium phase 3 trials were superior for islet transplantation alone (ITA) when compared to islet-after-kidney (IAK) transplantation. We set forth to evaluate if glycemic outcomes of ITA differ from those for IAK in 2 large cohorts obtained from the Collaborative Islet Transplant Registry (CITR).
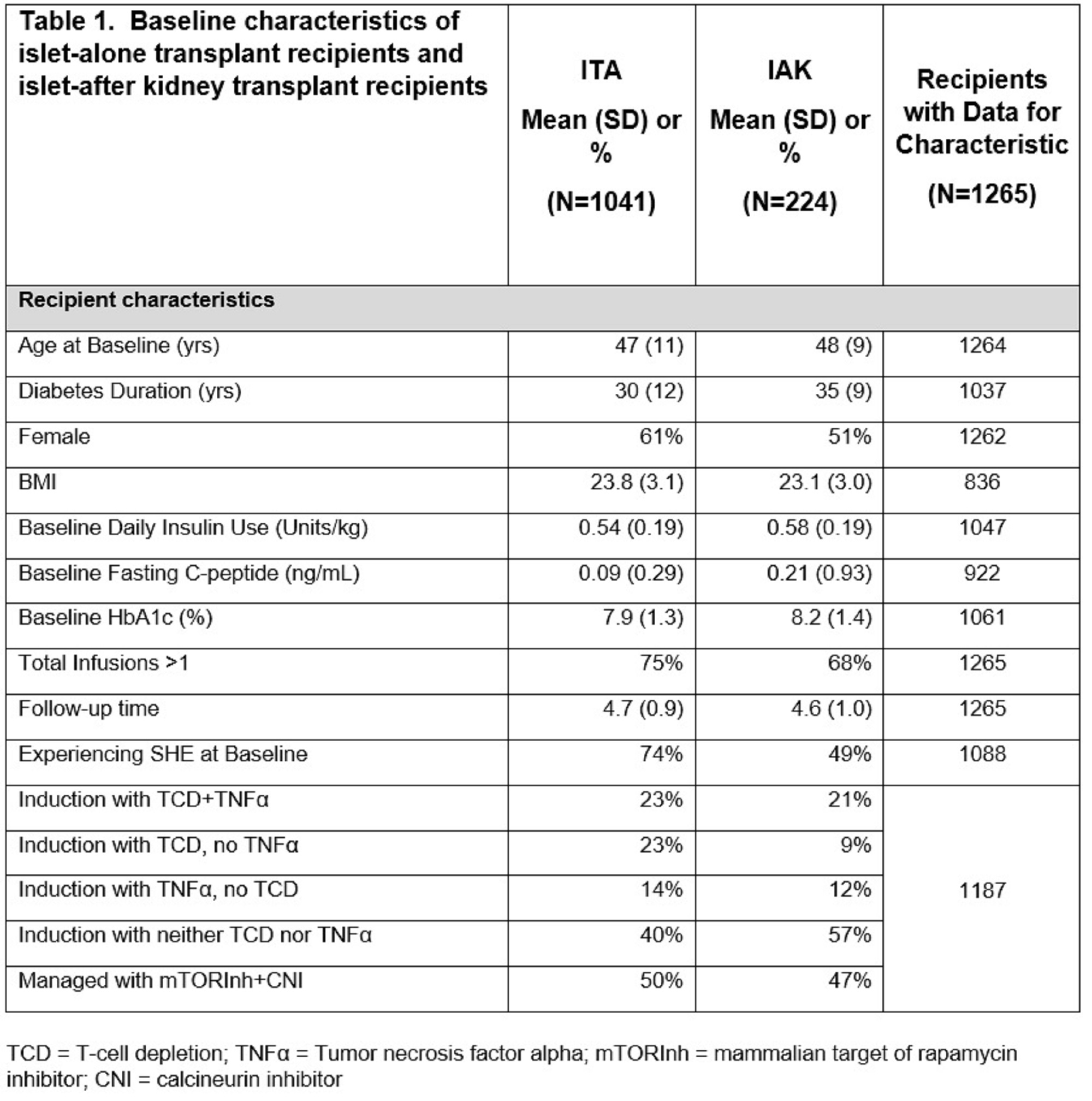
Methods: ITA (N=1041) and IAK (N=224) recipients registered in CITR with at least 1-year of follow-up reported as of February 15th, 2022, were analyzed. Mixed effects models were used to assess the univariate association of transplant type with outcomes including fasting glucose, HbA1c, severe hypoglycemic events (SHEs), insulin use, and C-peptide over 5 years following the last islet infusion. Immunosuppression strategy was dependent on the clinical protocol at each participating CITR site. Notably, a larger percentage of ITA participants underwent T-cell depletion (46 vs. 30%). Baseline characteristics for both study cohorts are shown in Table-1. Time in waiting list did not differ significantly between cohorts.
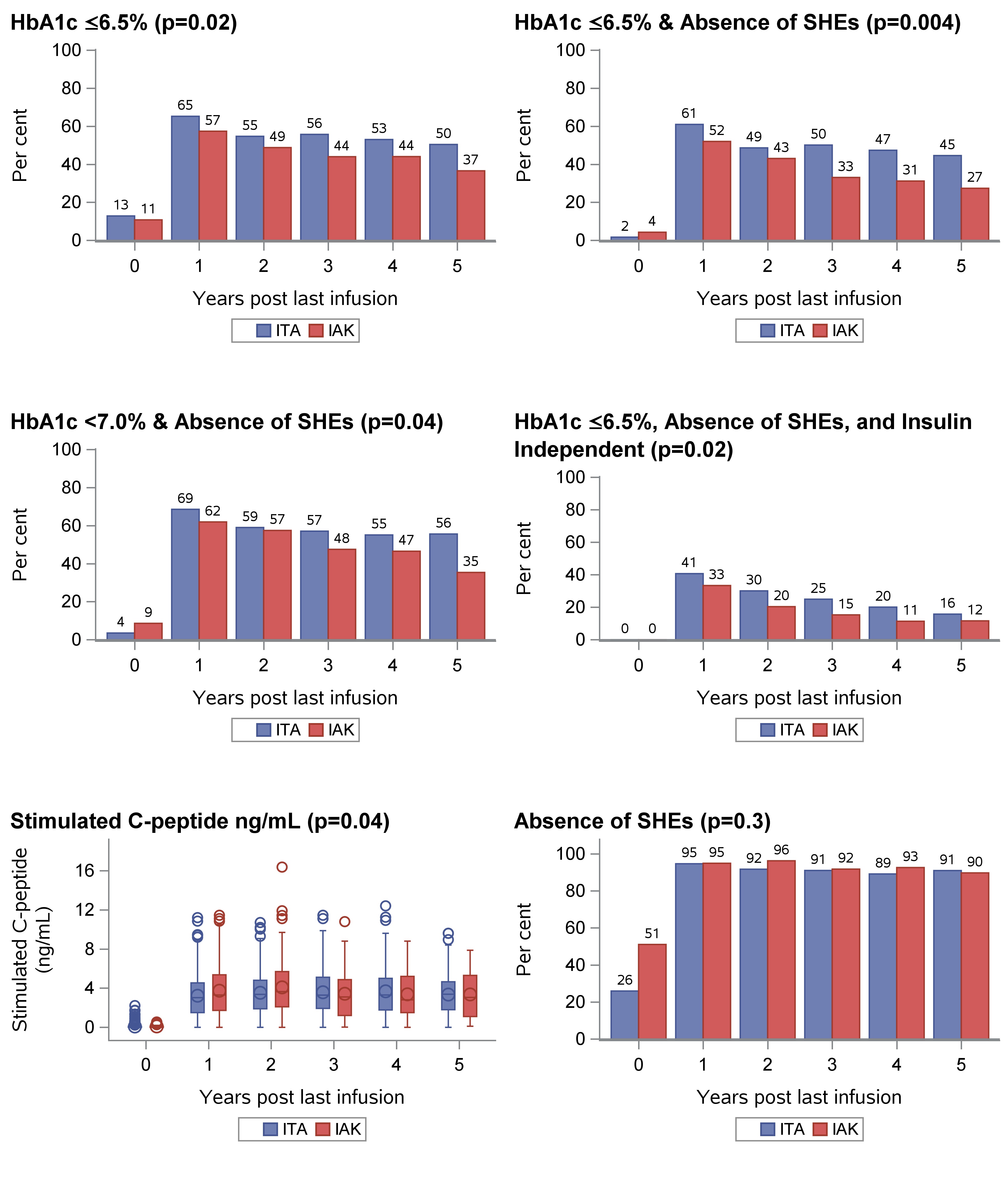
Results: The ITA cohort more often had HbA1c values ≤6.5% (p=0.02). By contrast, stimulated C-peptide was higher in the IAK group (p=0.04). No significant differences were found in fasting glucose, HbA1c <7.0%, absence of SHEs (ASHE), and insulin independence between the study cohorts. When evaluating more stringent glycemic outcomes, ITA performed better for the composite outcomes of HbA1c≤7.0% & ASHE (p=0.04), HbA1c≤6.5% & ASHE (p=0.004), and the optimal outcome of HbA1c≤6.5%, ASHE & insulin independence (p=0.02). Results are shown in Figure-1.
Conclusion: In these unadjusted analyses of glycemic outcomes, ITA recipients displayed better metabolic control defined by the combination of HbA1c, insulin independence and ASHE, compared to IAK. The higher stimulated C-peptide noted in the IAK recipients could be attributed to lower insulin sensitivity and/or lower eGFR, but this was not formally evaluated. Further analyses aimed at identifying factors predictive of optimal outcomes in each of these cohorts are required.
The CITR is funded by National Institute of Diabetes and Digestive and Kidney Diseases grants 1UCHDK098086-01 and 1UC4DK114839-01 and by JDRF International supplemental grants..