
Exploring preservation modalities in a split human pancreas model to investigate the effect on the islet isolation outcomes.
Antoine Buemi1,2, Nizar Mourad2, Caroline Bouzin4, Delphine Hoton3, Arnaud Devresse1, Tom Darius1,2, Nada Kanaan1, Pierre Gianello2, Michel Mourad1,2.
1Tansplant surgery, Cliniques Universitaires Saint Luc, Bruxelles, Belgium; 2Pole de Chirurgie experimentale, Université catholique de Louvain, Bruxelles, Belgium; 3Anathomopatholohy, Cliniques Universitaires Saint Luc, bruxelles, Belgium; 4IREC Imaging Platform, Université catholique de Louvain, Bruxelles, Belgium
Background: In islet transplantation, the use of dynamic hypothermic preservation methods is a current challenge, aiming to include in the the donor pool extended criteria pancreas without altering islet isolation results.
We here report a paired comparison of three pancreas preservation methods-cold storage (CS), hypothermic machine perfusion (HMP), and oxygenated machine perfusion (HMP O2)- using a split human pancreas model to assess the impact of the preservation method on islet function and isolation performance.
Methods: We used a human pancreas split model from discarded donors in which the pancreas head was preserved through the conventional CS method (“control group”) and the pancreas tail was preserved through three different preservation methods: CS, HMP, and HMP O2 (“study group”). After a same ischemia time in both head and tail of each organ, a separate islet isolation was performed in parallel. Donor characteristics, isolation data and functional tests results of isolated islets at one- and seven-day cultures of both segments were collected.
Results: Eight human pancreases were used in each group. Donor demographics, ischemia times and isolation data were similar in all groups (Table 1).
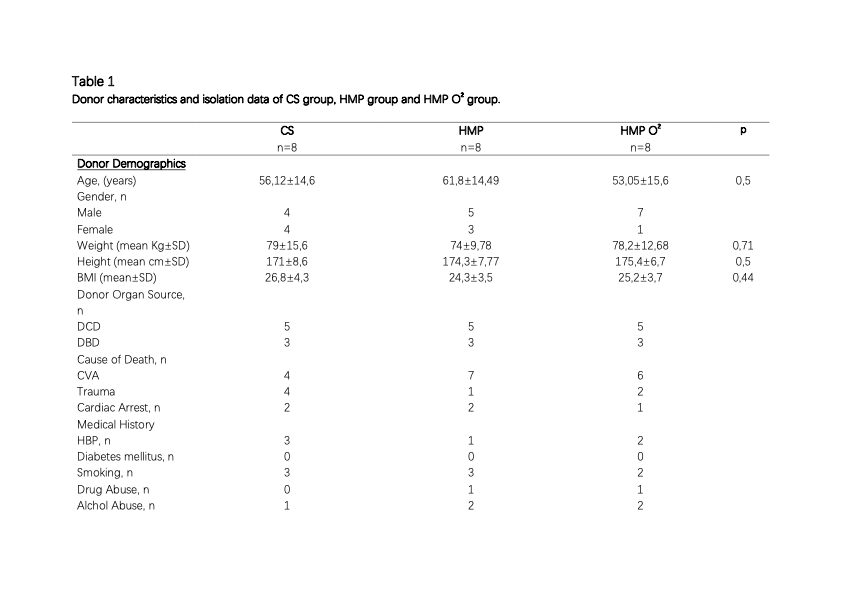
Compared to controls, IEQ/gr obtained was higher in both CS and HMP study groups (1219.77±844.3 IEQ vs 230±193 IEQ, p=0.006 and 1476.25±992.2 IEQ vs 251.33±195.8 IEQ, p=0.004 respectively). In contrast, the difference was not significant in the HMP O2 study group vs control (p=0.19) (Table 1, Figure 1).
No other significant paired isolation data differences were found.
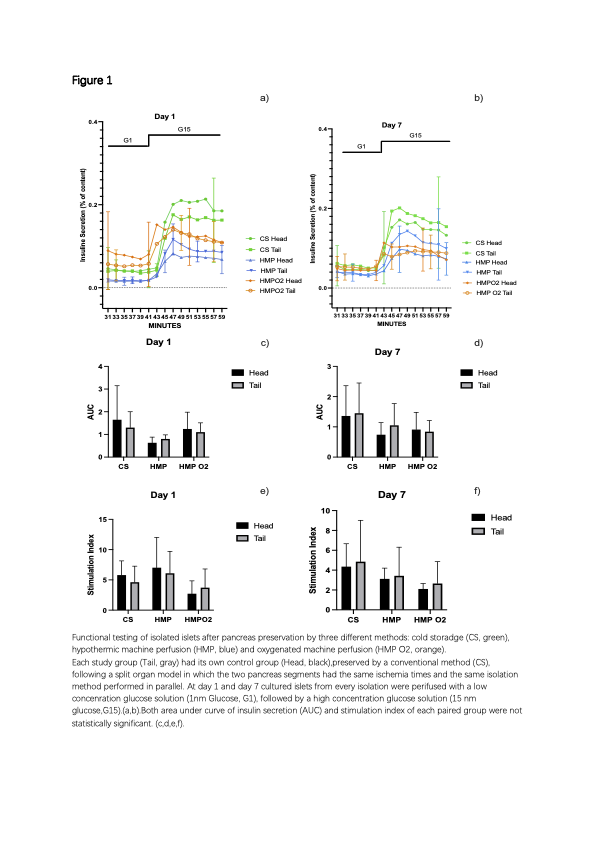
In each paired group (study vs control) and between the three study groups, functional testings of isolated islets were similar, including area under curve of insulin secretion and stimulation index (Table 1, Figure 1).
Conclusions: Our results suggest that all preservation methods show similar efficacy in terms of islet secretory function. Despite similar results in CS group and HMP group, the addition of a full oxygenation during the time of hypothermic machine preservation seems to negatively impact the performance on the islet isolation procedure.
[1] 1. Treckmann J, Moers C, Smits JM, et al. Machine perfusion
versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl Int. 2011; 24:548e554.
2. Dholakia S, Royston E., Friend P. et al. Preserving and perfusing the allograft pancreas: Past, present, and future.
Transplantation Reviews 32 (2018) 127–131
3. Taylor MJ, Baicu SC. Hypothermic perfusion of pancreas: Emphasis on preservation prior to islet isolation. In: Lee CY, Uygun K, eds. Methods in Bioengineering: Organ Preservation and Reengineering. Norwood, MA: Artech House; 2011:85e104.
4. Ricordi C, Lacy PE, Scharp DW 1989. Automated islet isolation from human pancreas. Diabetes 38: 140–142
5. Henquin J-C, Dufrane D, Kerr-Conte J 2015. Dynamics of glucose-induced insulin secretion in normal human islets Am J Physiol Endocrinol Metab 309: E640–E650, 2015.
6. Barnett M. J., McGhee-Wilson D., Shapiro A. M. J. 2004 Variation in Human Islet Viability Based on Different Membrane Integrity Stains. Cell Transplantation, Vol. 13, pp. 481–488, 2004
7. H.-S. Lee, J.-G. Lee 2016. The Introduction of Human Heme Oxygenase-1 and Soluble Tumor Necrosis Factor-a Receptor Type I With Human IgG1 Fc in Porcine Islets Prolongs Islet Xenograft Survival in Humanized Mice. Am J Transplantation 2016; 16: 44–57